Back to article: Mitochondria-Associated Membranes (MAMs) are involved in Bax mitochondrial localization and cytochrome c release
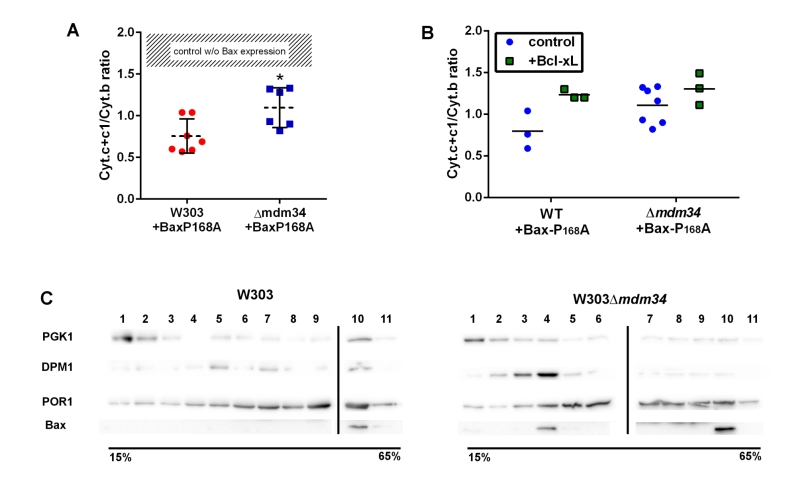
FIGURE 2: Bax-P168A is partly retained in ER, and has a decreased ability to release cytochrome c in κmdm34 cells. (A) Cytochrome c+c1/Cytochrome b ratios measured on mitochondria isolated from wild-type or κmdm34 cells expressing Bax-P168A. Each point represents a single mitochondria preparation. The hatched zone corresponds to the typical values found on mitochondria preparations from cells that do not express Bax [27][28][29][30][31][32][33][34][35][36][37][38][39]. *: p<0.05 (unpaired Student t-test). (B) Cytochrome c+c1/Cytochrome b ratios measured on mitochondria preparations isolated from strains co-expressing Bax-P168A and Bcl-xL. (C) Separation of whole extracts from cells expressing Bax-P168A on a 15-65% sucrose density gradient. Vertical lines mark the separation between different gels. Data are representative of four independent experiments.
27.
Arokium H, Camougrand N, Vallette FM, Manon S (2004). Studies of the interaction of substituted mutants of BAX with yeast mitochondria reveal that the C-terminal hydrophobic alpha-helix is a second ART sequence and plays a role in the interaction with anti-apoptotic BCL-xL. J Biol Chem 279(50): 52566-52573. 10.1074/jbc.M408373200
28.
Arokium H, Ouerfelli H, Velours G, Camougrand N, Vallette FM, Manon S (2007). Substitutions of potentially phosphorylatable serine residues of Bax reveal how they may regulate its interaction with mitochondria. J Biol Chem 282(48): 35104-35112. 10.1074/jbc.M704891200
29.
Renault TT, Teijido O, Missire F, Ganesan YT, Velours G, Arokium H, Beaumatin F, Llanos R, Athané A, Camougrand N, Priault M, Antonsson B, Dejean LM, Manon S. (2015). Bcl-xL stimulates Bax relocation to mitochondria and primes cells to ABT-737. Int J Biochem Cell Biol 64:136-146. 10.1016/j.biocel.2015.03.020
30.
Cartron PF, Arokium H, Oliver L, Meflah K, Manon S, Vallette FM (2005). Distinct domains control the addressing and the insertion of Bax into mitochondria. J Biol Chem 280(11): 10587-10598. 10.1074/jbc.M409714200
31. Simonyan L, Légiot A, Lascu I, Durand G, Giraud MF, Gonzalez C, Manon S (2017). The substitution of Proline 168 favors Bax oligomerization and stimulates its interaction with LUVs and mitochondria. Biochim Biophys Acta Biomembr 1859(6): 1144-1155. 10.1016/j.bbamem.2017.03.010
32. Garner TP, Reyna DE, Priyadarshi A, Chen HC, Li S, Wu Y, Ganesan YT, Malashkevich VN, Cheng EH, Gavathiotis E (2016). An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway. Mol Cell 63(3): 485-497. 10.1016/j.molcel.2016.06.010
33. Robin AY, Iyer S, Birkinshaw RW, Sandow J, Wardak A, Luo CS, Shi M, Webb AI, Czabotar PE, Kluck RM, Colman PM4 (2018). Ensemble Properties of Bax Determine Its Function. Structure 26(10): 1346-1359. 10.1016/j.str.2018.07.006
34. Burgess SM, Delannoy M, Jensen RE (1994). MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol 126(6): 1375-1391. 10.1083/jcb.126.6.1375
35. Berger KH, Sogo LF, Yaffe MP (1997). Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J Cell Biol 136(3): 545-553. 10.1083/jcb.136.3.545
36. Meisinger C, Rissler M, Chacinska A, Szklarz LK, Milenkovic D, Kozjak V, Schönfisch B, Lohaus C, Meyer HE, Yaffe MP, Guiard B, Wiedemann N, Pfanner N (2004) The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell 7(1): 61-71. 10.1016/j.devcel.2004.06.003
37. Meisinger C, Pfannschmidt S, Rissler M, Milenkovic D, Becker T, Stojanovski D, Youngman MJ, Jensen RE, Chacinska A, Guiard B, Pfanner N, Wiedemann N (2007). The morphology proteins Mdm12/Mmm1 function in the major beta-barrel assembly pathway of mitochondria. EMBO J 26(9): 2229-2239. 10.1038/sj.emboj.7601673
38. Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM (2004). Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279(20): 21085-21095. 10.1074/jbc.M400063200
39. Kale J, Kutuk O, Brito GC, Andrews TS, Leber B, Letai A, Andrews DW (2018). Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep 19(9): e45235. 10.15252/embr.201745235