Research Reports:
Microbial Cell, Vol. 10, No. 6, pp. 133 - 140; doi: 10.15698/mic2023.06.798
Atg1, a key regulator of autophagy, functions to promote MAPK activation and cell death upon calcium overload in fission yeast
1 Laboratory of Molecular Pharmacogenomics, Department of Pharmaceutical Sciences, Faculty of Pharmacy, Kindai University, Higashi-Osaka, 577-8502, Japan.
2 Laboratory of Hygienic Science, Department of Pharmacy, Hyogo Medical University, Kobe, 650-8530, Japan.
Keywords: Pmk1 MAPK, autophagy, cell death, fission yeast, calcium tolerance.
Abbreviations:
WT – wild-type,
vic – viability in the presence of immunosuppressant and Cl−,
CFUs – colony-forming units,
MIC – Minimum Inhibitory Concentration.
Received originally: 26/10/2022 Received in revised form: 21/04/2023
Accepted: 26/04/2023
Published: 10/05/2023
Correspondence:
Reiko Sugiura, Laboratory of Molecular Pharmacogenomics, Department of Pharmaceutical Sciences, Faculty of Pharmacy, Kindai University, Higashi-Osaka, 577-8502, Japan; Phone: +81-6-4307-3642; Fax: +81-6-6730-1394; sugiurar@phar.kindai.ac.jp
Conflict of interest statement:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Please cite this article as: Teruaki Takasaki, Ryosuke Utsumi, Erika Shimada, Asuka Bamba, Kanako Hagihara, Ryosuke Satoh, and Reiko Sugiura (2023). Atg1, a key regulator of autophagy, functions to promote MAPK activation and cell death upon calcium overload in fission yeast. Microbial Cell 10(6): 133-140. doi: 10.15698/mic2023.06.798
Abstract
Autophagy promotes or inhibits cell death depending on the environment and cell type. Our previous findings suggested that Atg1 is genetically involved in the regula-tion of Pmk1 MAPK in fission yeast. Here, we showed that Δatg1 displays lower levels of Pmk1 MAPK phosphorylation than did the wild-type (WT) cells upon treatment with a 1,3-β-D-glucan synthase inhibitor micafungin or CaCl2, both of which activate Pmk1. Moreover, the overproduction of Atg1, but not that of the kinase inactivating Atg1D193A activates Pmk1 without any extracellular stimuli, suggesting that Atg1 may promote Pmk1 MAPK signaling activation. Notably, the overproduction of Atg1 induces a toxic effect on the growth of WT cells and the deletion of Pmk1 failed to suppress the cell death induced by Atg1, indicating that the Atg1-mediated cell death requires additional mechanism(s) other than Pmk1 activation. Moreover, atg1 gene deletion induces tolerance to micafungin and CaCl2, whereas pmk1 deletion induces severe sensitivities to these compounds. The Δatg1Δpmk1 double mutants display intermediate sensitivities to these compounds, showing that atg1 deletion partly suppressed growth inhibition induced by Δpmk1. Thus, Atg1 may act to promote cell death upon micafungin and CaCl2 stimuli regardless of Pmk1 MAPK activity. Since micafungin and CaCl2 are intracellular calcium inducers, our data reveal a novel role of the autophagy regulator Atg1 to induce cell death upon calcium overload independent of its role in Pmk1 MAPK activation.
INTRODUCTION
Autophagy is a universal mechanism to maintain cellular homeostasis in eukaryotic cells through protein degradation and organelle turnover, which is considered to contribute to energy generation and cell survival [1]. However, increasing evidence suggests that autophagy also plays a key role in cell death depending on the cellular context or environmental conditions [2][3].
–
We have previously shown that the deletion of the atg1+ gene, encoding a serine/threonine kinase essential for autophagy, induced phenotypes shared by the deletion mutants of the components of the Pmk1 MAPK signaling pathway, a fission yeast orthologue of the ERK MAPK pathway [4]. These phenotypes include the vic phenotype and the suppression of the growth inhibition induced by overexpression of Pck2, an upstream regulator of Pmk1 MAPK. The vic phenotype represents “viability in the presence of immunosuppressant and Cl−”, a shared phenotype associated with deletion or inhibition of the components of the Pmk1 MAPK signaling pathway. Deletion of the atg1+ gene also suppressed the growth inhibition associated with the overexpression of Pck2, an upstream activator of Pmk1. It should be mentioned that the degree of the vic phenotype observed in the Δatg1 mutant is weaker than that of the Δpmk1 mutant [4]. These data suggested that although Atg1 functions in the Pmk1 signaling pathway, the contribution may be weaker than that of MAPKK or MAPKKK.
–
Here, we conducted biochemical analyses and obtained data consistent with the role of Atg1 in promoting Pmk1 MAPK signaling activation, including the impact of the deletion and overexpression of atg1+ on Pmk1 phosphorylation levels. Unexpectedly, overexpression of atg1+ induces cell death, which cannot be suppressed by Pmk1 deletion. Furthermore, Δatg1 mutants display tolerance to Ca2+-increasing stimuli, micafungin, and Ca2+, in contrast to the severe sensitivity observed in Δpmk1. Overexpression of Atg1 confers cell death both in the WT and Pmk1 deletion background, indicating that Atg1 and Pmk1 do not function in a linear fashion in cell death. Our findings reveal additional roles of Atg1 in mediating the cell death response upon calcium overload in the vegetative condition.
RESULTS
Identification of Atg1 as an activator of Pmk1 MAPK signaling
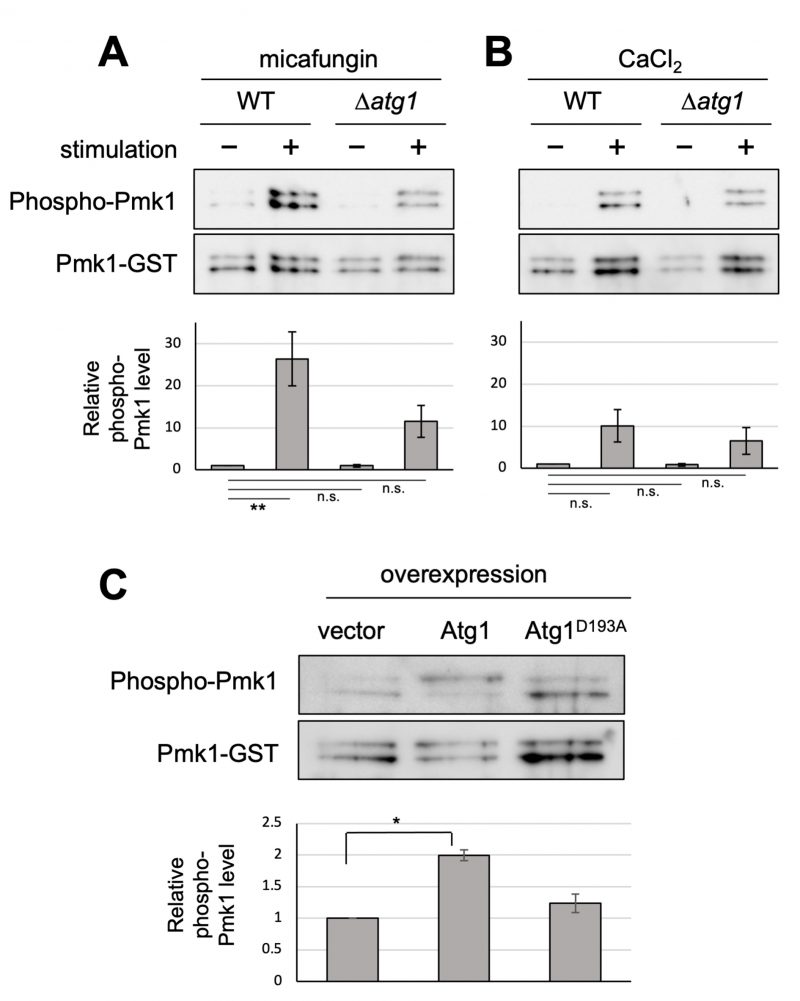
Our previous genetic screen revealed that the deletion of the atg1+ gene displayed the vic phenotype [4]. Here, we further characterized Δatg1 mutants to obtain clues regarding the roles of Atg1 in the vegetative stage. To investigate if Atg1 can affect Pmk1 MAPK signaling activation, Pmk1 MAPK phosphorylation levels were investigated in atg1 deletion cells in comparison with the WT cells. The basal Pmk1 phosphorylation status was not significantly affected in the atg1 deletion cells (Figure 1A). We then treated cells with micafungin, a non-competitive inhibitor of 1,3-β-glucan synthase, which has been reported to stimulate cell integrity MAPK signaling [5]. Micafungin treatment significantly increased Pmk1 phosphorylation levels in the WT cells, and this induction of Pmk1 MAPK activation was impaired in atg1 deletion cells (Figure 1A). Since micafungin was shown to induce intracellular Ca2+ concentrations [6][7], we next investigated if the addition of CaCl2 can affect Pmk1 MAPK activation. 0.2 M CaCl2 also stimulated Pmk1 MAPK activation, and the deletion of atg1 impaired the rise in the Pmk1 phosphorylation (Figure 1B). Thus, Atg1 may be required for facilitating MAPK activation in response to cell integrity-damaging stimuli and calcium stimuli.
–
We next examined the effect of the overexpression of Atg1. The WT cells overexpressing Atg1 displayed significantly higher levels of Pmk1 MAPK phosphorylation, in the absence of any stimuli, as compared with the cells harboring the control vector alone (Figure 1C). Notably, overexpression of Atg1D193A, the kinase-dead mutant of Atg1 [8], did not induce Pmk1 activation (Figure 1C), indicating that this induction of Pmk1 MAPK activation is dependent on the kinase activity of Atg1. We confirmed that the levels of overexpression of the WT and kinase-dead Atg1D193A are approximately equal (Figure S1), indicating that the differences in the levels of Pmk1 activation associated with the overexpression of the WT Atg1 or the kinase-dead Atg1D193A are derived from the Atg1 kinase activity. Thus, these data are consistent with the genetic interaction that Atg1 acts upstream of Pmk1 MAPK to promote MAPK activation.
–
The role of Atg1 as a mediator of cell death in response to calcium overload
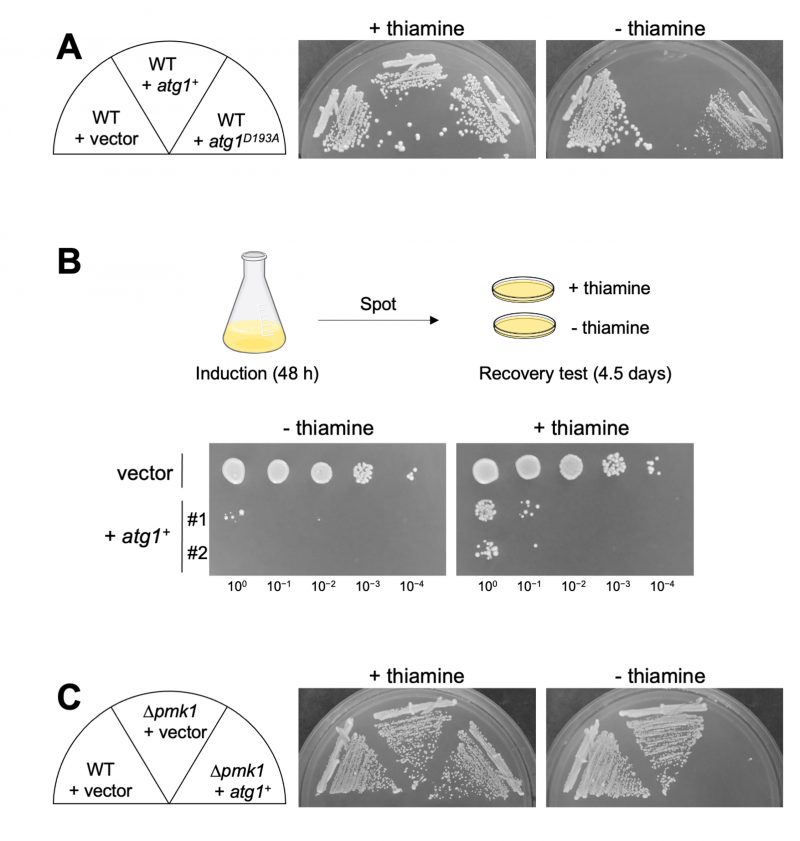
We further investigated the impact of Atg1 overexpression on cell growth. Since the hyperactivation of Pmk1 MAPK signaling leads to cell growth inhibition [9], Atg1 overexpression may exert the same effect by promoting Pmk1 MAPK signaling activation. The growth of the WT cells expressing atg1+ was much slower than that of the WT cells harboring the control vector alone, indicating that Atg1 induces a reduction of cell growth when overproduced (Figure 2A). The growth reduction was also observed when the kinase-inactivating atg1D193A was overexpressed, although the effect was modest as compared with that induced by the WT atg1+. To determine whether or not cell death is occurring or if growth is simply inhibited upon Atg1 overexpression, we measured colony-forming units (CFUs) by spotting cells on the EMM plates with or without thiamine after 48 hours of induction of Atg1 overexpression (Figure 2B). The results showed that the viability of the cells subjected to Atg1 overexpression is quite low even when plated in the repressed condition, showing approximately less than 1% of cell viability as compared with that of the cells harboring the control vector alone. Thus, the reduction of cell growth induced by Atg1 overexpression was irreversible, indicating that Atg1 overexpression causes cell death rather than growth inhibition. Notably, the pmk1 deletion did not suppress the cell death induced by Atg1 overexpression, indicating that the Atg1-mediated cell death does not require Pmk1 MAPK activation (Figure 2C).
–
To further obtain clues regarding the Atg1-mediated cell death, we investigated if cell death by Atg1 overexpression can be suppressed by the deletion of other Atg genes. If the deletion of Atg genes may affect the toxicity induced by Atg1 overexpression, the downstream of cell death by Atg1 overexpression is not Pmk1, but the Atg pathway. Among the strains deleted for each of the 13 Atg genes that we examined, none of them suppressed the Atg1 overexpression-induced cell death (Figure S2). We, therefore, consider that the Atg pathway, as a whole, is not required for the execution of cell death triggered by Atg1.
–
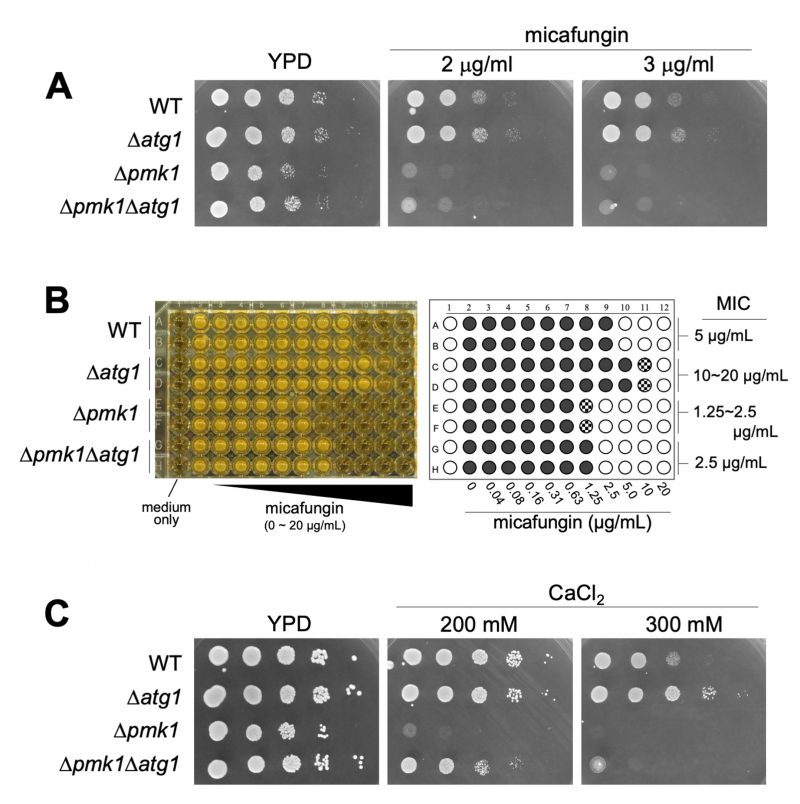
Next, we investigated the phenotypic consequence of the Atg1 deletion on micafungin sensitivity as the loss-of-function mutants or deletion of the components of the Pmk1 MAPK signaling pathway shared the phenotype of micafungin hypersensitivity. These components include Pmk1, Pek1 MAPKK, Mkh1 MAPKKK, Pck2, Rho2, Cpp1 farnesyl transferase, and Cwg2 geranylgeranyl transferase [9][10][11]. Surprisingly, atg1 deletion cells displayed tolerance to micafungin, in contrast to the severe sensitivity to the compound associated with Δpmk1. As shown in Figure 3A, the growth of Δatg1 was better than that of the WT cells in the presence of micafungin, whereas the growth of Δpmk1 was severely inhibited by the compound. Thus, although genetic and biochemical experiments suggest that Atg1 acts upstream of Pmk1 MAPK signaling, atg1 deletion did not induce micafungin hypersensitivity but rather enhanced tolerance to micafungin. To further characterize the phenotypic discrepancies between Δatg1 and Δpmk1 cells in terms of micafungin tolerance, we next analyzed the effect of the pmk1 deletion on micafungin resistance elicited by atg1 deletion. The Δatg1Δpmk1 double mutant cells displayed an intermediate sensitivity to micafungin as the double mutants grew faster than Δpmk1 cells and slower than Δatg1 cells in the medium containing micafungin (Figure 3A).
–
To evaluate the difference in the micafungin sensitivities between the single and the double mutant strains more quantitatively, we analyzed the growth of each strain against a wide range of concentrations of micafungin using a 96-well plate format with a liquid medium and determined the Minimum Inhibitory Concentration (MIC) of micafungin in each strain. As shown in Figure 3B, the MIC in Δatg1 cells is 10∼20 μg/ml, apparently higher than that in the WT cells (5 μg/ml). Moreover, the MIC in Δpmk1 cells (1.25∼2.5 μg/ml) is lower than that in the WT cells and higher than that in the Δpmk1Δatg1 double mutant strain (2.5 μg/ml). Thus, pmk1 deletion partly suppressed micafungin resistance associated with atg1 deletion, suggesting that the tolerance of atg1 deletion to the growth inhibition by micafungin is mediated not only by Pmk1 signaling but also by additional signaling mechanism(s). To further analyze the phenotypes of Δatg1, we investigated the effect of the atg1 deletion on the sensitivity to calcium, which also stimulates Pmk1 activation. Again, the growth of Δatg1 was better than that of the WT cells in the YPD medium containing 0.2 M CaCl2, whereas the growth of Δpmk1 was severely inhibited in the same medium (Figure 3C). The Δatg1Δpmk1 double mutant displayed an intermediate sensitivity to CaCl2 (Figure 3C), again showing that the suppression by pmk1 deletion is partial. We also examined the CFU assay using the YES medium to evaluate the micafungin and CaCl2 sensitivities and obtained similar results to that obtained with the YPD medium (Figure S3). Thus, Atg1 functions to hamper cell growth in the presence of CaCl2 and micafungin regardless of Pmk1 MAPK activity.
–
DISCUSSION
Here, we provide evidence that the fission yeast Atg1 regulates cell death responses upon intracellular calcium load in addition to its role in promoting Pmk1 MAPK.
–
First, Atg1 was shown to positively regulate Pmk1 MAPK activation based on the findings that Atg1 deletion inhibits Pmk1 MAPK phosphorylation upon micafungin and CaCl2 stimuli and that Atg1 overproduction stimulates Pmk1 phosphorylation. These data are consistent with our previous genetic data showing that atg1 deletion phenotypically recapitulates deletion mutants of the upstream components of the Pmk1 MAPK pathway [4]. In mammals, Martinez-Lopez et al. proposed an unconventional function of ATG proteins in the regulation of ERK phosphorylation by showing that deleting Atg7 or Atg5 or blocking LC3 lipidation decreases ERK phosphorylation [12]. Intriguingly, components of the ERK signaling cascade are associated with autophagosomes, proposing that autophagosomes serve as scaffolds or cellular platforms regulating ERK phosphorylation [12][13]. Our previous study also showed the involvement of the autophagy system as a whole in the regulation of MAPK, because some autophagy-related gene deletions phenotypically recapitulate MAPK signaling inhibition [4]. It is noteworthy that Atg1 overexpression promotes Pmk1 signaling in a kinase-dependent manner. It has been reported that Atg1 kinase organizes autophagosome formation by phosphorylating Atg9 in budding yeast [14]. Thus, the concept of autophagosome formation initiated by Atg1 as a mechanism to regulate ERK phosphorylation via spatial coordination of ERK signaling may be universal. Whether other autophagy-related genes also facilitate Pmk1 MAPK activation, or Atg1 specifically functions to initiate MAPK signaling stimulation via its kinase activity would be an important issue for future study.
–
Secondly, we characterized the cell death phenotypes mediated by Atg1. Cell death stimulation was observed when Atg1 is overexpressed in WT cells. The failure of pmk1 deletion to suppress the cell death associated with the overexpression of Atg1 implicates that the Atg1-mediated cell death response is not solely explained by Pmk1 MAPK activation. Further phenotypic analysis showed that atg1 deletion induced tolerance to micafungin and CaCl2 in contrast to the severe sensitivities of pmk1 deletion to these agents. Micafungin and CaCl2 shared the property to stimulate intracellular calcium concentrations and calcium signaling [6]. Thus, Atg1 plays a key role to mediate cell death in response to the two calcium-stimulating stresses, whereas Pmk1 is required for proliferation during the same stresses. Thus, it seems plausible to hypothesize that the cell death system involving Atg1 is executed in cells with maladaptation to calcium-increasing stimuli. Importantly, the cell death execution mediated by Atg1 was observed both in the WT and pmk1 deletion background, again suggesting that Atg1 mediates cell death independently of Pmk1 MAPK activity. It should be noted that Atg1 overexpression confers toxicity even in cells deleted for each of the Atg pathway genes. These results suggest that neither Pmk1 nor the Atg pathway serves as a downstream target of Atg1-mediated cell death. We then investigated if the calcium tolerance phenotype associated with the atg1 deletion is shared by other Atg pathway deletion cells. The results showed that many of the genes deleted for the Atg pathway exhibited similar levels of calcium tolerance to that of the atg1 deletion cells (Figure S4). These results suggest that not only Atg1 but also the Atg pathway at large seems to be involved in the calcium-related cell death process. However, this will not exclude the possibility that Atg1 overexpression induces some proteostasis stress responses which may trigger cell death irrelevant to the Atg pathway. Intriguingly, caspofungin, another candin fungicidal compound that also inhibits 1,3-β-D-glucan synthase, has been reported to induce programmed cell death in Saccharomyces cerevisiae and Candida albicans [15][16]. In addition, micafungin induced apoptosis in Candida parapsilosis [17]. Therefore, the cell death response elicited by micafungin may be executed by apoptosis machinery in fission yeast. Micafungin and other echinocandin class of antifungal drugs have assumed an increasingly vital role in the therapy of invasive candidiasis [18]. Although autophagy often accompanies cell death following various toxic insults, autophagy-dependent cell death is highly contextual [19]. In this regard, elucidating the cell death mechanism mediated by Atg1 in response to micafungin may contribute to proposing a novel strategy for anti-fungal treatment.
MATERIALS AND METHODS
Yeast strains, media, and molecular biology
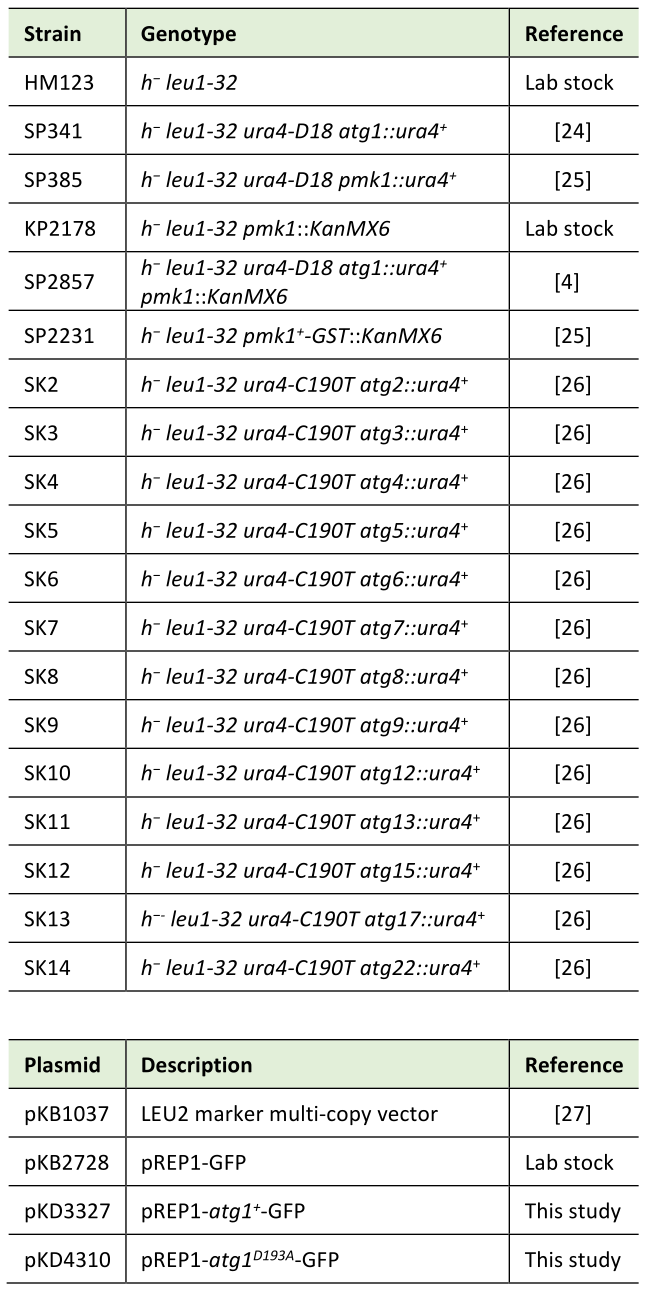
Schizosaccharomyces pombe strains and plasmids used in this study are listed in Table 1. The complete medium YPD or YES, and the minimal medium EMM have been described previously [20][24]. Standard yeast culture and genetic methods were used except where noted [21][22]. Kinase-defective variant of Atg1 (Atg1D193A) was generated by inverse PCR using Vent DNA Polymerase (NEB) with the sense primer (#2144) 5′-TAGCAGCCTTTGGTTTTGCACGTTACCTTCAAACGTC-3′ and the reverse primer (#2130) 5′-GGTAACGTGCAAAACCAAAGGCTGCTAATTTAAGCAT-3′.
–
Table 1. Strains and plasmids used in this study. |
 |
Purification and Detection of phosphorylated Pmk1
Cells expressing GST-tagged Pmk1 from the endogenous pmk1+ promoter were suspended in ice cold lysis buffer [1% Triton X, 30 mM Tris-HCl (pH 8.0), 2 mM EDTA, 0.1% 2-mercaptoethanol plus protease inhibitor mixtures and phosphatase inhibitor mixtures] and homogenized with glass beads using Multi-beads Shocker (Yasui Kikai, Osaka, Japan). The lysates were cleared by centrifugation at 15,000 rpm for 15 min, and GST-tagged Pmk1 was purified by glutathione agarose beads. The purified proteins were resolved in 10% SDS-PAGE gels and transferred to the PVDF membrane (Millipore). Phosphorylated GST-Pmk1 was immunoreacted with either rabbit anti-phospho-p42/44 antibodies (Cell Signaling) or rabbit anti-phospho-Pmk1 antibodies [23] and anti-rabbit-IgG HRP-conjugated secondary antibody (Cell Signaling), then developed with Chemi-Lumi One Super (Nacalai tesque, Japan). The blots were stripped using WB stripping solution (Nacalai tesque) and reprobed with rabbit anti-GST antibodies [23] to measure the amount of total GST-Pmk1 loaded in each lane. Protein levels were quantified using MULTI GAUGE Ver. 3.2 software (Fujifilm, Japan)
–
MIC assay
Strains were grown to mid-log phase in liquid YES at 27°C and adjusted to OD660 = 0.5, and then diluted 150-fold with the fresh medium. 100 µl of aliquots were pipetted into a 96-well plate and mixed with the equal amout of YES medium containing the different concentrations (2-fold serial dilutions) of micafungin. Plates were incubated at 27°C for 3 days.
–
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). We have run a one-way ANOVA, followed by a post hoc test using Dunnett’s test for multiple comparisons. The Welch’s two sample t-test was used to determine the level of significance of differences between the two groups. Statistical significance was defined as p<0.05.
REFERENCES
- Mizushima N, and Komatsu M (2011). Autophagy: Renovation of Cells and Tissues. Cell. 147(4): 728–741. 10.1016/j.cell.2011.10.026
- Gozuacik D, and Kimchi A (2007). Autophagy and Cell Death. Curr Top Dev Biol. 78: 217–245. 10.1016/s0070-2153(06)78006-1
- Doherty J, and Baehrecke EH (2018). Life, death and autophagy. Nat Cell Biol. 20(10): 1110–1117. 10.1038/s41556-018-0201-5
- Takasaki T, Utsumi R, Shimada E, Tomimoto N, Satoh R, and Sugiura R (2022). Autophagy-related genes genetically interact with Pmk1 MAPK signaling in fission yeast. Micropublication Biology. 2022. 10.17912/micropub.biology.000618
- Takada H, Nishimura M, Asayama Y, Mannse Y, Ishiwata S, Kita A, Doi A, Nishida A, Kai N, Moriuchi S, Tohda H, Giga-Hama Y, Kuno T, and Sugiura R (2007). Atf1 is a target of the mitogen-activated protein kinase Pmk1 and regulates cell integrity in fission yeast. Molecular biology of the cell. 18(12): 4794–4802. 10.1091/mbc.e07-03-0282
- Deng L, Sugiura R, Takeuchi M, Suzuki M, Ebina H, Takami T, Koike A, Iba S, and Kuno T (2006). Real-Time Monitoring of Calcineurin Activity in Living Cells: Evidence for Two Distinct Ca2+-dependent Pathways in Fission Yeast. Mol Biol Cell. 17(11): 4790–4800. 10.1091/mbc.e06-06-0526
- Ogata F, Satoh R, Kita A, Sugiura R, and Kawasaki N (2017). Evaluation of a novel method for measurement of intracellular calcium ion concentration in fission yeast. J Toxicol Sci. 42(2): 159–166. 10.2131/jts.42.159
- Pan Z-Q, Shao G-C, Liu X-M, Chen Q, Dong M-Q, and Du L-L (2020). Atg1 kinase in fission yeast is activated by Atg11-mediated dimerization and cis-autophosphorylation. Elife. 9: e58073. 10.7554/elife.58073
- Ma Y, Kuno T, Kita A, Asayama Y, and Sugiura R (2006). Rho2 Is a Target of the Farnesyltransferase Cpp1 and Acts Upstream of Pmk1 Mitogen-activated Protein Kinase Signaling in Fission Yeast. Mol Biol Cell. 17(12): 5028–5037. 10.1091/mbc.e06-08-0688
- Doi A, Kita A, Kanda Y, Uno T, Asami K, Satoh R, Nakano K, and Sugiura R (2015). Geranylgeranyltransferase Cwg2?Rho4/Rho5 module is implicated in the Pmk1 MAP kinase?mediated cell wall integrity pathway in fission yeast. Genes Cells. 20(4): 310–323. 10.1111/gtc.12222
- Ma Y, Kuno T, Kita A, Nabata T, Uno S, and Sugiura R (2006). Genetic Evidence for Phospholipid-Mediated Regulation of the Rab GDP-Dissociation Inhibitor in Fission Yeast. Genetics. 174(3): 1259–1271. 10.1534/genetics.106.064709
- Martinez-Lopez N, Athonvarangkul D, Mishall P, Sahu S, and Singh R (2013). Autophagy proteins regulate ERK phosphorylation. Nature communications. 4: 2799. 10.1038/ncomms3799
- Martinez-Lopez N, and Singh R (2014). ATGs: Scaffolds for MAPK/ERK signaling. Autophagy. 10(3): 535–537. 10.4161/auto.27642
- Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, Maiolica A, Hansmann I, Pfaffenwimmer T, Kijanska M, Stoffel I, Lee SS, Brezovich A, Lou JH, Turk BE, Aebersold R, Ammerer G, Peter M, and Kraft C (2014). Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Molecular cell. 53(3): 471–483. 10.1016/j.molcel.2013.12.011
- Hao B, Cheng S, Clancy CJ, and Nguyen MH (2013). Caspofungin Kills Candida albicans by Causing both Cellular Apoptosis and Necrosis. Antimicrob Agents Ch. 57(1): 326–332. 10.1128/aac.01366-12
- Chin C, A U of MS of M 55 Lake Ave N, Worcester, MA 01655, U S, A D-FCI 44 Binney St, Boston, MA 02115, U S, Donaghey F, Helming K, McCarthy M, Rogers S, and Austriaco N (2014). Deletion of AIF1 but not of YCA1/MCA1 protects Saccharomyces cerevisiae and Candida albicans cells from caspofungin-induced programmed cell death. Microb Cell. 1(2): 58. 10.15698/mic2014.01.119
- Shirazi F, Lewis RE, and Kontoyiannis DP (2015). Micafungin induced apoptosis in Candida parapsilosis independent of its susceptibility to micafungin. Microb Cell. 2(11): 445–450. 10.15698/mic2015.11.236
- Gonzalez-Lara M, and Ostrosky-Zeichner L (2020). Invasive Candidiasis. Semin Resp Crit Care. 41(01): 003–012. 10.1055/s-0040-1701215
- Denton D, and Kumar S (2019). Autophagy-dependent cell death. Cell Death Differ. 26(4): 605–616. 10.1038/s41418-018-0252-y
- Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E, Sugiura R, and Kuno T (1996). The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol. 16(12): 6752–64. 10.1128/mcb.16.12.6752
- Rose MD, Winston F, and Hieter. and P (1990). Methods in yeast genetics: A laboratory course manual. Cold Spring Harbor Laboratory Press. 197(1): 273–274. 10.1016/0307-4412(91)90039-B
- Alfa C, Fantes P, Hyams J, and Warbrich M and E (1993). Experiments with fission yeast: A laboratory course manual. Trends Genet. 10.1016/0962-8924(94)90127-9
- Sugiura R, Toda T, Dhut S, Shuntoh H, Kuno T, and Kuno T (1999). The MAPK kinase Pek1 acts as a phosphorylation-dependent molecular switch. Nature. 399(6735): 479–483. 10.1038/20951
- Bimbó A, Jia Y, Poh SL, Karuturi RKM, Elzen N den, Peng X, Zheng L, O’Connell M, Liu ET, Balasubramanian MK, and Liu J (2005). Systematic deletion analysis of fission yeast protein kinases. Eukaryot Cell. 4(4): 799–813. 10.1128/ec.4.4.799-813.2005
- Kanda Y, Satoh R, Takasaki T, Tomimoto N, Tsuchiya K, Tsai CA, Tanaka T, Kyomoto S, Hamada K, Fujiwara T, and Sugiura R (2021). Sequestration of the PKC ortholog Pck2 in stress granules as a feedback mechanism of MAPK signaling in fission yeast. J Cell Sci. 134(2): jcs250191. 10.1242/jcs.250191
- Mukaiyama H, Kajiwara S, Hosomi A, Giga-Hama Y, Tanaka N, Nakamura T, and Takegawa K (2009). Autophagy-deficient Schizosaccharomyces pombe mutants undergo partial sporulation during nitrogen starvation. Microbiology+. 155(12): 3816–3826. 10.1099/mic.0.034389-0
- Ma Y, Sugiura R, Saito M, Koike A, Sio SO, Fujita Y, Takegawa K, and Kuno T (2007). Six new amino acid-auxotrophic markers for targeted gene integration and disruption in fission yeast. Curr Genet. 52(2): 97–105. 10.1007/s00294-007-0142-1
–
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
We thank Dr. Mohan K. Balasubramanian for providing the atg1 knockout strain, Dr. Kaoru Takegawa for providing the strains lacking autophagy-related genes, and the members of Sugiura Lab for discussion and technical support. This study was supported by the MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (S1411037), and by JSPS KAKENHI Grant Numbers 20K07058 (T. Takasaki), JP20K06494 (R. Satoh), JPS1411037 (R. Sugiura). This work was also supported by a grant by the Antiaging Project for Private Universities (R. Sugiura).
COPYRIGHT
© 2023

Atg1, a key regulator of autophagy, functions to promote MAPK activation and cell death upon calcium overload in fission yeast by Takasaki et al. is licensed under a Creative Commons Attribution 4.0 International License.