Research Reports:
Microbial Cell, Vol. 1, No. 1, pp. 37 - 42; doi: 10.15698/mic2014.01.122
Early manifestations of replicative aging in the yeast Saccharomyces cerevisiae.
1 Faculty of Bioengineering and Bioinformatics, Moscow State University, Vorobyevy Gory 1, Moscow, Russia
2 Belozersky Institute of Physico-Chemical Biology, Moscow State University, Vorobyevy Gory 1, Moscow, Russia
3 Institute of Mitoengineering, Moscow State University, Vorobyevy Gory 1, Moscow, Russia
Keywords: yeast, aging, stress resistance, retrograde signaling.
Received originally: 22/11/2013 Received in revised form: 10/12/2013
Accepted: 12/12/2013
Published: 06/01/2014
Correspondence:
Fedor F. Severin, Belozersky Institute of Physico-Chemical Biology, Moscow State University, Vorobyevy Gory 1; Moscow 119992, Russia severin@belozersky.msu.ru
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Maksim I. Sorokin, Dmitry A. Knorre, and Fedor F. Severin (2014). Early manifestations of replicative aging in the yeast Saccharomyces cerevisiae. Microbial Cell 1(1): 37-42.
Abstract
The yeast Saccharomyces cerevisiae is successfully used as a model organism to find genes responsible for lifespan control of higher organisms. As functional decline of higher eukaryotes can start as early as one quarter of the average lifespan, we asked whether S. cerevisiae can be used to model this manifestation of aging. While the average replicative lifespan of S. cerevisiae mother cells ranges between 15 and 30 division cycles, we found that resistances to certain stresses start to decrease much earlier. Looking into the mechanism, we found that knockouts of genes responsible for mitochondria-to-nucleus (retrograde) signaling, RTG1 or RTG3, significantly decrease the resistance of cells that generated more than four daughters, but not of the younger ones. We also found that even young mother cells frequently contain mitochondria with heterogeneous transmembrane potential and that the percentage of such cells correlates with replicative age. Together, these facts suggest that retrograde signaling starts to malfunction in relatively young cells, leading to accumulation of heterogeneous mitochondria within one cell. The latter may further contribute to a decline in stress resistances.
INTRODUCTION
The budding yeast Saccharomyces cerevisiae is an example of a unicellular organism with markedly asymmetrical division: newly emerged daughter cells are normally significantly smaller than the mothers (see [1]). However, the asymmetry is not limited to the differences in volume: mother-to-daughter transport of a specific set of mRNAs prevents mating type switching by repression of the HO promoter in the daughter cells [2]. Further, it was shown that carbonylated or misfolded proteins and extrachromosomal rDNA circles [3] are retained in the mother cells. Recently, we showed that such transport is necessary to prevent high stress susceptibility of the daughter cells [4]. At the same time, multiple rounds of asymmetrical divisions result in subsequent decrease in viability in replicatively old cells. As a result, the chance of a spontaneous death of the yeast cell increases with each division cycle after production of approximately 15 buds [5], [6]. One of the most pronounced consequences of asymmetrical redistribution of cytoplasmic contents appears to be the mitochondrial morphology. In yeast, abnormal morphology of the mitochondrial network and increased reactive oxygen species production can be observed already after 6-8 divisions [7]. The latter is not surprising because the mother/daughter redistribution of mitochondria is not random: the mother cell tends to retain mitochondria with higher superoxide level [8], while mitochondria with intact aconitase (a Fe-S cluster-containing enzyme that is sensitive to ROS) are preferentially transported to the bud [9].
–
Here we report a number of changes in cellular physiology (i.e. a decrease in stress resistances) that occur even earlier than 6-8 cell divisions. We also found that the probability of harboring a mitochondrial fragment with significantly different transmembrane potential than in the rest of the mitochondrial network within same cell increases with each cell cycle. These early age-dependent changes might significantly affect the stress resistances of the cells.
RESULTS
Stress resistances change early during replicative aging
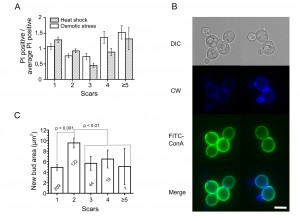
First, to test the age-dependence of stress resistances, exponentially growing yeast cells were subjected to a number of treatments (Table 1) and then stained with propidium iodide (PI) to visualize dead cells and with calcofluor white (CW) to visualize birth and bud scars. Then we counted the scars for each dead and live cell and calculated the percentage of PI positive cells for each aging class, where age = 1 corresponds to virgin daughter cells (with birth scars only), age = 2 corresponds to mother cells (with one birth scar and one bud scar), etc. As the proportion of cells with more than four scars did not exceed 6% of the total (Figure S1), we pooled them into one group (≥5) and analyzed it as a single aging class. We found that in case of heat shock the dead/alive cell ratios for different aging classes were statistically different (Figure 1A). The same results were obtained for hyperosmotic (Figure 1A) and acidic stresses (Figure S2). However, in the case of oxidative stress (menadione) and for stresses induced by high concentrations of ethanol or butanol, there were no statistically significant differences (Figure S2).
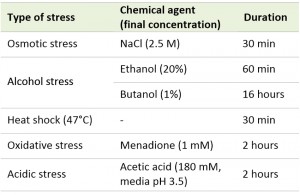 | TABLE 1. Types of stresses used in this study. |
Virgin daughter cells have some unique properties. In particular, daughter cells have increased duration of G1 phase of cell cycle compared to mother cells [10] and demonstrate significantly lower resistances to acetic acid stress and heat shock [4]. Therefore, we excluded the daughter cells (age = 1) from the calculations and performed the statistical Kruskal–Wallis test for the mother cells only. It was found that for heat shock and hyperosmotic stress the p value is below 0.01 (Figure 1A), whereas for acetic acid stress the p value was 0.29 (Figure S2).
Obviously the ability to extrude propidium iodide is not the only criterion of stress resistance. Another is the ability to start growing after the return to stress-free conditions. Thus, we decided to test whether this ability depends on replicative age. To do that, we synchronized yeast cells with nocodazole and stained the cell walls with FITC-ConA (Concanavalin A labeled with green fluorescent dye). FITC-ConA irreversibly binds to the cell thus allowing to distinguish between the old cells (stained) and the newly emerged (unstained ones). Then the cells were subjected to a mild stress: +42°C for 30 minutes. After removal of the stress, we incubated the cells at 30°C for 90 minutes, stained them with calcofluor white to visualize ages, and measured the sizes of the newly formed (FITC-ConA free) buds (Figure 1B). It appeared that cells with two scars have larger buds compared to the other age classes (Figure 1C).
–
Together these data support the idea that the stress resistances of yeast cells which represent the major part of the population (ages 1-5) vary depending on their replicative age.
–
Mitochondrial function and stress resistance during replicative aging
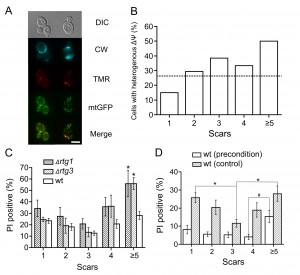
What determines these early age-dependent changes in the stress resistances? It is known that mitochondrial morphology of the mother cells starts to change already after five or six cell cycles [7]. Thus, we decided to look in more detail at age-dependent changes in mitochondria. We used yeast cells expressing mitochondria-targeted GFP [11] and stained them with tetramethylrhodamine (TMR). TMR is a lipophilic cation that accumulates in mitochondria depending on their ΔΨ. We noticed that the mother cells have much higher levels of heterogeneity within a cell than the daughters and that the level of heterogeneity positively correlates with replicative age (Figures 2 A, B).
What are the possible links between the mitochondrial heterogeneity and the stress resistances? Malfunctioning mitochondria in yeast induce specific transcriptional changes in the nuclei that act to normalize the mitochondrial activities. Therefore, our finding that aging yeast cells contain heterogeneous mitochondria (meaning that some of them might be not in optimal conditions) may reflect the fact that this signaling pathway in such cells is compromised. The mitochondria-to-nucleus signaling is driven by transcription factors of the retrograde pathway: RTG1, RTG2, and RTG3 [12]. We found that the deletion of rtg1 or rtg3 affects old cells but not the young ones when subjected to severe heat stress (30 minutes at 47°C, Figure 2C). This indicates that cells with replicative age higher than four are more reliant on retrograde signaling.
–
We also compared the effects of preconditioning on the stress responses of cells of different ages. It appeared that the preconditioning increased survival of the young cells to such an extent, that proportion of PI-positive cells became similar between the cells with 1 to 4 scars (Figure 2D). The viability of the preconditioned cells with ≥5 scars also seemed to be higher than in control. However, the proportion of PI-positive cells of this aging class appeared to be different from the one for younger ages (Figure 2D). This supports the idea that in the old cells the ability to adapt to a changing environment is diminished.
–
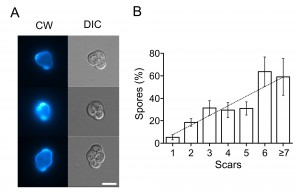
Does this mean that the mitochondrial heterogeneity is a sign of a general decline of the cell? Recently we showed that differentiation of yeast cells requires two subpopulations of mitochondria [13]. This result implies that the ability of cells to differentiate in response to environmental signals increases with replicative age. To test this, we induced meiosis in diploid cells by plating them on solid medium containing 2% potassium acetate. As predicted, it appeared that the ability of cells to form spores positively correlates with age (Figure 3); at the same time, the diploid cells show similar stress resistance profile as haploids cell (Figure S3). It should be noted, however, that the latter effect could be due to correlation of sporulation efficiency and cell size [14], which also strongly correlates with cell age (Figure S4).
DISCUSSION
Our data suggest that the response to environmental challenges of yeast starts to change early during their replicative aging. In particular, these changes lead to a decreased resistance to certain stresses of the cells with 4-5 scars. This implies that under natural (as opposed to laboratory) conditions the cells are unlikely to reach replicative age of twenty or thirty. Does this mean that most of the works on yeast replicative aging (which were conducted under laboratory conditions) were studying laboratory artifacts? It was found that similar groups of genes regulate replicative lifespan in yeast and longevity in worms and mice (histone deacetylases, antioxidant enzymes, etc., see for review [15]). At the same time, similarly to yeast, functional decline of humans also starts at relatively early age. For example, a decrease in the number of immune T-cells in humans starts at the age of 15 years [16]. Therefore, our data suggest that yeast can serve as a model to study not only lifespan-regulating mechanisms, but also the mechanisms of early age-associated functional decline. Interestingly, fission yeast display similar aging phenotype. Recently, it was reported that while S. pombe does not seem to age replicatively under standard laboratory conditions, stresses force them to do so [17].
–
Our data also show that not all stress resistances show sharp age-dependence. Moreover, the ability for spore formation seems to increase during early aging. In other words, early aging in yeast displays features of both regular aging and differentiation. At the same time, it is well known that heterogeneity of individual cells in clonal microbial populations helps them to survive in randomly changing environment. For instance, a small fraction of bacterial cells (persisters) show slower growth rate and, at the same time, increased antibiotic resistance [18]. Early age-dependent differentiation of yeast cells has also been already reported. In the stationary phase of growth, S. cerevisiae forms two types of cells: quiescent and nonquiescent [19], [20]. Quiescent cells are represented mainly by virgin daughters; they are arrested in the G1 phase of the cell cycle and have a higher chance to survive in the stationary phase. The nonquiescent cells are continuing to proliferate. Our data suggest that such differentiation is more complex than simply mother-daughter differences. Possibly, there is a distinct number (higher than two) of aging classes of cells, with each class showing a unique set of resistances to harsh conditions.
MATERIALS AND METHODS
Strains and growth conditions
In this study, we used strains of W303 genetic background strain (Table 2). If not indicated, exponentially growing yeast cells were prepared as following: prior to the treatments the cells were grown overnight at 30°C on solid YPD medium prepared according to [21] and transferred in liquid YPD for 3.5-4 hours until the optical density (OD550) of the suspension reached 0.2 (4 x 106 cells per ml).
 | TABLE 2. Yeast strains used in this study. [13], [22] |
Stress induction
Cell suspensions in liquid YPD were subjected to various types of stresses: 2.5 M NaCl for 30 minutes, 20% ethanol for 1 hour, 1% butanol for 16 hours, heat shock (47°C) for 30 minutes, 1 mM menadione for 2 hours, or 180 mM acetic acid (pH of the medium was set to 3.5) for 2 hours. The types of stresses used in this study are summarized in Table 1. Incubation of the cells for 30 minutes at 37°C was used as preconditioning for heat shock. Sporulation of diploid W303 cells was induced on solid potassium acetate-containing medium (2% potassium acetate, 2% agar) for 3 days.
–
New bud formation
The cells were synchronized with nocodazole (10 μg/ml), washed 4 times with YP, then moved to YPD containing FITC-conjugated Concanavalin A (FITC-ConA) and incubated at 30°C for 10 minutes and then at 42°C for 30 minutes. Then the cells were washed 2 times with YPD and incubated at 30°C in YPD for 90 minutes. After 90 minutes, the cells were transferred to YP media containing calcofluor white, as described in the Microscopy section.
–
Microscopy
After the stresses the cells were washed with YP containing Propidium Iodide (PI, 1 µg/ml) and calcofluor white (CW, 5 µM). PI-positive cells were counted as dead. Calcofluor white was used to visualize scars on the cell surface (birth scar and bud scars) for replicative age measurements. Cell viability, replicative age, and percent of spores were determined manually using an Olympus BX51 microscope. Photographs were taken with a DP30BW CCD camera. Cell size was measured manually using the ImageJ program.
–
Statistical analysis
All data are presented as average and standard error. Wilcoxon signed ranked unpaired test and Kruskal-Wallis test were used to compare datasets from different strains or conditions with the R software package. All viability experiments were performed independently 4 times, and each time at least 90 cells were analyzed unless indicated otherwise. When the proportions of the PI-positive cells were low, we counted the age distribution of PI-positive cells only and then extrapolated the result using the average survival in this experiment.
References
- S. Di Talia, H. Wang, J.M. Skotheim, A.P. Rosebrock, B. Futcher, and F.R. Cross, "Daughter-Specific Transcription Factors Regulate Cell Size Control in Budding Yeast", PLoS Biology, vol. 7, pp. e1000221, 2009. http://dx.doi.org/10.1371/journal.pbio.1000221
- N. Bobola, R. Jansen, T.H. Shin, and K. Nasmyth, "Asymmetric Accumulation of Ash1p in Postanaphase Nuclei Depends on a Myosin and Restricts Yeast Mating-Type Switching to Mother Cells", Cell, vol. 84, pp. 699-709, 1996. http://dx.doi.org/10.1016/S0092-8674(00)81048-X
- D.A. Sinclair, and L. Guarente, "Extrachromosomal rDNA Circles— A Cause of Aging in Yeast", Cell, vol. 91, pp. 1033-1042, 1997. http://dx.doi.org/10.1016/S0092-8674(00)80493-6
- D.A. Knorre, I.A. Kulemzina, M.I. Sorokin, S.A. Kochmak, N.A. Bocharova, S.S. Sokolov, and F. F. Severin, "Sir2-dependent daughter-to-mother transport of the damaged proteins in yeast is required to prevent high stress sensitivity of the daughters", Cell Cycle, vol. 9, pp. 4501-4505, 2010. http://dx.doi.org/10.4161/cc.9.22.13683
- K. Ashrafi, D. Sinclair, J.I. Gordon, and L. Guarente, "Passage through stationary phase advances replicative aging in Saccharomyces cerevisiae", Proceedings of the National Academy of Sciences, vol. 96, pp. 9100-9105, 1999. http://dx.doi.org/10.1073/pnas.96.16.9100
- B.K. Kennedy, N.R. Austriaco, and L. Guarente, "Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span.", The Journal of cell biology, vol. 127, pp. 1985-1993, 1994. http://dx.doi.org/10.1083/jcb.127.6.1985
- Y.T. Lam, M.T. Aung-Htut, Y.L. Lim, H. Yang, and I.W. Dawes, "Changes in reactive oxygen species begin early during replicative aging of Saccharomyces cerevisiae cells", Free Radical Biology and Medicine, vol. 50, pp. 963-970, 2011. http://dx.doi.org/10.1016/j.freeradbiomed.2011.01.013
- J.R. McFaline‐Figueroa, J. Vevea, T.C. Swayne, C. Zhou, C. Liu, G. Leung, I.R. Boldogh, and L.A. Pon, "Mitochondrial quality control during inheritance is associated with lifespan and mother–daughter age asymmetry in budding yeast", Aging Cell, vol. 10, pp. 885-895, 2011. http://dx.doi.org/10.1111/j.1474-9726.2011.00731.x
- H. Klinger, M. Rinnerthaler, Y.T. Lam, P. Laun, G. Heeren, A. Klocker, B. Simon-Nobbe, J.R. Dickinson, I.W. Dawes, and M. Breitenbach, "Quantitation of (a)symmetric inheritance of functional and of oxidatively damaged mitochondrial aconitase in the cell division of old yeast mother cells", Experimental Gerontology, vol. 45, pp. 533-542, 2010. http://dx.doi.org/10.1016/j.exger.2010.03.016
- P.G. Lord, and A.E. Wheals, "Variability in individual cell cycles of Saccharomyces cerevisiae.", Journal of cell science, 1981. http://www.ncbi.nlm.nih.gov/pubmed/7033253
- B. Westermann, and W. Neupert, "Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae.", Yeast (Chichester, England), 2000. http://www.ncbi.nlm.nih.gov/pubmed/11054823
- Z. Liu, and R.A. Butow, "Mitochondrial Retrograde Signaling", Annual Review of Genetics, vol. 40, pp. 159-185, 2006. http://dx.doi.org/10.1146/annurev.genet.40.110405.090613
- A.N. Starovoytova, M.I. Sorokin, S.S. Sokolov, F.F. Severin, and D.A. Knorre, "Mitochondrial signaling inSaccharomyces cerevisiaepseudohyphae formation induced by butanol", FEMS Yeast Research, vol. 13, pp. 367-374, 2013. http://dx.doi.org/10.1111/1567-1364.12039
- G.R. Calvert, and I.W. Dawes, "Cell size control of development in Saccharomyces cerevisiae", Nature, vol. 312, pp. 61-63, 1984. http://dx.doi.org/10.1038/312061a0
- B.M. Wasko, and M. Kaeberlein, "Yeast replicative aging: a paradigm for defining conserved longevity interventions", FEMS Yeast Research, vol. 14, pp. 148-159, 2013. http://dx.doi.org/10.1111/1567-1364.12104
- G. PAWELEC, and A. LARBI, "Immunity and ageing in man: Annual review 2006/2007", Experimental Gerontology, 2007. http://dx.doi.org/10.1016/j.exger.2007.09.009
- M. Coelho, A. Dereli, A. Haese, S. Kühn, L. Malinovska, M. DeSantis, J. Shorter, S. Alberti, T. Gross, and I. Tolić-Nørrelykke, "Fission Yeast Does Not Age under Favorable Conditions, but Does So after Stress", Current Biology, vol. 23, pp. 1844-1852, 2013. http://dx.doi.org/10.1016/j.cub.2013.07.084
- N.Q. Balaban, J. Merrin, R. Chait, L. Kowalik, and S. Leibler, "Bacterial Persistence as a Phenotypic Switch", Science, vol. 305, pp. 1622-1625, 2004. http://dx.doi.org/10.1126/science.1099390
- C. Allen, S. Büttner, A.D. Aragon, J.A. Thomas, O. Meirelles, J.E. Jaetao, D. Benn, S.W. Ruby, M. Veenhuis, F. Madeo, and M. Werner-Washburne, "Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures", The Journal of Cell Biology, vol. 174, pp. 89-100, 2006. http://dx.doi.org/10.1083/jcb.200604072
- A.D. Aragon, A.L. Rodriguez, O. Meirelles, S. Roy, G.S. Davidson, P.H. Tapia, C. Allen, R. Joe, D. Benn, and M. Werner-Washburne, "Characterization of Differentiated Quiescent and Nonquiescent Cells in Yeast Stationary-Phase Cultures", Molecular Biology of the Cell, vol. 19, pp. 1271-1280, 2008. http://dx.doi.org/10.1091/mbc.E07-07-0666
- F. Sherman, "[1] Getting started with yeast", Guide to Yeast Genetics and Molecular Biology, pp. 3-21, 1991. http://dx.doi.org/10.1016/0076-6879(91)94004-V
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
We are very grateful to Svyatoslav Sokolov, Vitaly Kushnirov, Boris Feniouk, and Peter Kamenski for a valuable discussion of our work. This work was supported by the Russian Foundation for Basic Research grant 12-04-01412-а.
COPYRIGHT
© 2013

Early manifestations of replicative aging in the yeast Saccharomyces cerevisiae. by Maksim I. Sorokin et al. is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.