Research Articles:
Microbial Cell, Vol. 1, No. 6, pp. 179 - 188; doi: 10.15698/mic2014.06.150
Heat shock protein 90 and calcineurin pathway inhibitors enhance the efficacy of triazoles against Scedosporium prolificans via induction of apoptosis
Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas M.D. Anderson Cancer Center, Houston, TX 77030, U.S.A.
Keywords: apoptosis, 17AAG, calcineurin, itraconazole, posaconazole, reactive oxygen species.
Received originally: 12/03/2014 Received in revised form: 13/05/2014
Accepted: 20/05/2014
Published: 02/06/2014
Correspondence:
Dimitrios P. Kontoyiannis, Department of Infectious Diseases, Infection Control and Employee Health, Unit 402, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard; Houston, TX 77030, USA dkontoyi@mdanderson.org
Conflict of interest statement:
D.P.K has received research support and honoraria from Pfizer, Astellas Pharma US, and Merck and Co. Inc, F.S reports no conflicts.
Please cite this article as: Fazal Shirazi and Dimitrios P. Kontoyiannis (2014). Heat shock protein 90 and calcineurin pathway inhibitors enhance the efficacy of triazoles against Scedosporium prolificans via induction of apoptosis. Microbial Cell 1(6): 179-188.
Abstract
Scedosporium prolificans is a pathogenic mold resistant to current antifungals, and infection results in high mortality. Simultaneous targeting of both ergosterol biosynthesis and heat shock protein 90 (Hsp90) or the calcineurin pathway in S. prolificans may be an important strategy for enhancing the potency of antifungal agents. We hypothesized that the inactive triazoles posaconazole (PCZ) and itraconazole (ICZ) acquire fungicidal activity when combined with the calcineurin inhibitor tacrolimus (TCR) or Hsp90 inhibitor 17-demethoxy-17-(2-propenylamino) geldanamycin (17AAG). PCZ, ICZ, TCR and 17AAG alone were inactive in vitro against S. prolificans spores (MICs > 128 μg/ml). In contrast, MICs for PCZ or ICZ in combination with TCR or 17AAG (0.125-0.50 μg/ml) were much lower compared with drug alone. In addition PCZ and ICZ in combination with TCR or 17AAG became fungicidal. Because apoptosis is regulated by the calcineurin pathway in fungi and is under the control of Hsp90, we hypothesized that this synergistic fungicidal effect is mediated via apoptosis. This observed fungicidal activity was mediated by increased apoptosis of S. prolificans germlings, as evidenced by reactive oxygen species accumulation, decreased mitochondrial membrane potential, phosphatidylserine externalization, and DNA fragmentation. Furthermore, induction of caspase-like activity was correlated with TCR or 17AAG + PCZ/ICZ-induced cell death. In conclusion, we report for the first time that PCZ or ICZ in combination with TCR or 17AAG renders S. prolificans exquisitely sensitive to PCZ or ICZ via apoptosis. This finding may stimulate the development of new therapeutic strategies for patients infected with this recalcitrant fungus.
INTRODUCTION
Scedosporium prolificans is an emerging filamentous fungus that causes severe, frequently fatal pulmonary or disseminated opportunistic infections in immunocompromised patients [1]. S. prolificans is inherently resistant to treatment with a wide range of antifungals, including the new generation of broad-spectrum triazoles [1][2][3][4]. Hence, new therapeutic strategies for Scedosporium infections are urgently needed.
–
In pathogenic fungi, the calcineurin pathway and heat shock protein 90 (Hsp90) play major roles in maintaining fungal homeostatic cell responses, including resistance to antifungal agents [5][6][7][8][9][10]. The calcineurin inhibitor tacrolimus (TCR) is an immunosuppressive agent widely used in solid organ and hematopoietic stem cell transplant recipients to prevent graft rejection [11]. TCR binds to the intracellular protein immunophilin FKB12 and forms a complex, thereby inhibiting activation of the calcineurin pathway. In vitro studies have suggested synergy between triazoles and calcineurin inhibitors against Aspergillus spp. and the Mucorales [12][13][14]. Our group recently reported that treatment with the combination of TCR and posaconazole (PCZ) improves control of invasive, necrotizing cutaneous mucormycosis in immunosuppressed mice compared with PCZ alone [15].
–
Hsp90 is a molecular chaperone involved in stress responses of Candida albicans and Aspergillus spp. and plays a major role in echinocandin resistance via regulation of the calcineurin pathway [6][16]. Specifically, pharmacological inhibition of Hsp90 by 17-demethoxy-17-(2-propenylamino) geldanamycin (17AAG) prevents azole resistance and abrogates this resistance in C. albicans and A. fumigatus in a human host [6][16]. In addition, researchers recently suggested a role for the calcineurin pathway in regulation of apoptosis in fungi [17][18]. However, the role of Hsp90 in apoptosis remains unclear. Therefore, simultaneous targeting of both ergosterol biosynthesis and Hsp90 or calcineurin pathways in S. prolificans may be an important strategy for restoring the potency of antifungal agents. Specifically, we hypothesized that TCR or 17AAG in combination with the triazoles PCZ or itraconazole (ICZ) induces apoptosis in S. prolificans. Thus, we examined the effects of TCR and 17AAG co-administration on PCZ and ICZ activity using several in vitro methods to evaluate induction of apoptosis in S. prolificans.
RESULTS
PCZ and ICZ are inactive when used alone against S. prolificans, but exhibit significant fungicidal activity when combined with TCR or 17AAG
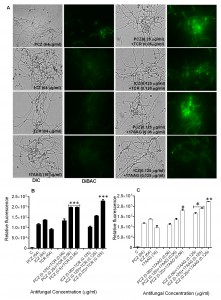
Individually, PCZ, ICZ, TCR, and 17AAG were inactive against S. prolificans (isolates 1 to 3), with minimum inhibitory concentrations (MICs) ranging from 32 to 128 μg/ml. In contrast, the combination of PCZ or ICZ with either TCR or 17AAG rendered S. prolificans exquisitely more sensitive to the triazoles than did use of the triazoles alone (Table 1). Specifically, in combination with TCR or 17AAG, PCZ and ICZ were synergistic, with a fractional inhibitory concentration index (ΣFIC) of 0.5. In addition, bis-[1,3-dibutylbarbituric acid] trimethine oxonol (DiBAC) vital staining revealed enhanced uptake of stain and plasma membrane damage in S. prolificans germlings (isolates 1 and 2) exposed to PCZ or ICZ in combination with TCR or 17AAG (Figure 1 A-C; Table S1). Use of PCZ or ICZ (0.125-0.25 μg/ml) in combination with TCR or 17AAG resulted in 2.0- to 2.5-fold greater plasma membrane damage than did the use of triazoles alone.
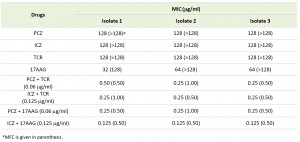 | TABLE 1. In vitro antimicrobial activity of PCZ and ICZ in combination with TCR or 17AAG against S. prolificans isolates. |
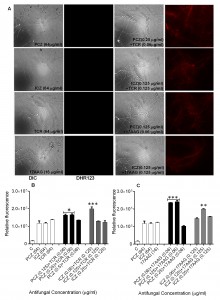
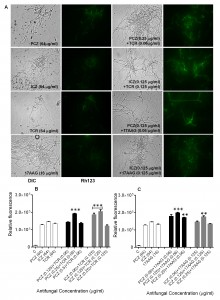
Detection of intracellular Reactive Oxygen Species (ROS) accumulation and loss of mitochondrial membrane potential (ΔΨm) in S. prolificans (isolates 1 and 2) germlings in response to treatment with PCZ or ICZ combined with TCR or 17AAG
Staining of S. prolificans germlings with dihydrorhodamine (DHR)-123 (red fluorescence) and rhodamine (Rh)-123 (green fluorescence) was most prominent in germlings treated with PCZ or ICZ in combination with TCR or 17AAG (Figures 2 and 3). A small percentage of control germlings and germlings treated with PCZ or ICZ alone exhibited positive staining for DHR-123 and Rh-123 (Figures 2 and 3). Staining with DHR123 and Rh-123 increased markedly when triazoles were combined with TCR or 17AAG, respectively (1.2-2.1 fold increase in fluorescence intensity), compared with triazoles alone (Figures 2 and 3 A-C). Isolate 2 in particular, had 1.0-2.1 fold and 1.3-2.1 fold increase in fluorescence for ROS accumulation and loss of mitochondrial potential, respectively, over germlings treated with triazoles alone (Table S1). Accumulation of intracellular ROS and disruption of ΔΨm are important steps in mitochondria-mediated apoptosis. These data indicate that treatment with PCZ or ICZ combined with TCR or 17AAG can trigger apoptosis in S. prolificans due to accumulation of ROS.
Evidence of apoptosis in S. prolificans (isolates 1 and 2) induced by treatment with PCZ or ICZ in combination with TCR or 17AAG
Because various drugs can induce both apoptosis and necrosis in mammalian cells [19], we sought to differentiate between apoptotic and necrotic S. prolificans protoplast using annexin V-fluorescein isothiocyanate (FITC)–propidium iodide (PI) double staining, in which apoptotic cells are stained with annexin V-FITC (green), whereas the nuclei of necrotic cells are stained with PI (red) [20][21][22]. Incubation of S. prolificans (isolate 1) protoplasts in the presence of PCZ (0.25 μg/ml) or ICZ (0.125 μg/ml) in combination with TCR (0.060-0.125 μg/ml) at 37°C for 3 h led to annexin V-FITC staining of 35-50% of the protoplasts. We found that 30-40% of protoplasts exhibited annexin V-FITC staining, when incubated with PCZ or ICZ in combination with 17AAG (0.060-0.125 μg/ml) (Table 2). In S. prolificans isolate 2, however, 40-65% of protoplasts were apoptotic after incubation with PCZ or ICZ in combination with TCR, and 35-70% were apoptotic after incubation with PCZ or ICZ with 17AAG (Table S1). We observed no annexin V-FITC staining in untreated protoplasts (Table 2). These results suggested that a fungicidal property of PCZ and ICZ was due to induction of apoptosis in S. prolificans cells, especially in combination with TCR or 17AAG.
TABLE 2. Percentage of S. prolificans (isolate 1) cells stained with annexin V, TUNEL and PI for detection of phosphatidylseriene exposure, DNA fragmentation and cell membrane integrity respectively.
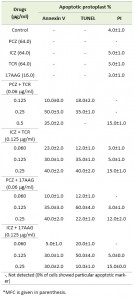
To confirm the apoptotic features of PCZ and ICZ in S. prolificans germlings, we evaluated nuclear DNA fragmentation using a terminal deoxynucelotidyl transferase dUTP nick end labeling (TUNEL) assay. S. prolificans germlings exposed to PCZ or ICZ for 3 h at 37°C exhibited marked nuclear DNA fragmentation in a concentration-dependent manner (Table 2). The proportion of TUNEL-positive germlings was higher in both isolate 1 (50-60%) and isolate 2 (30-60%) in the presence of PCZ or ICZ (0.125 μg/ml) combined with 17AAG than when combined with TCR (isolate 1, 20-40%; isolate 2, 35-55%) (Tables 2 and S1).
–
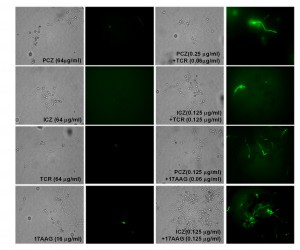
Induction of caspase-like activity in S. prolificans (isolate 1) germlings treated with PCZ or ICZ in combination with TCR or 17AAG
Caspases are activated in the early stages of apoptosis and play a central role in the apoptotic cascade [23][24]. Although caspases are not present in fungi, researchers have identified orthologs of mammalian caspases, called metacaspases in fungi (25). We stained S. prolificans germlings (isolate 1) pretreated with PCZ or ICZ in combination with TCR or 17AAG with the cell-permeable, broad-spectrum caspase inhibitor CaspACE-Z-VAD-FMK. In this staining, a green fluorescent signal is a direct measure of the amount of active caspase in a cell. S. prolificans germlings with activated metacaspases, treated with azoles in combination with TCR or 17 AAG were stained green, whereas germlings exposed to azoles alone remained unstained. This result indicated that treatment with PCZ or ICZ plus TCR or 17AAG triggered an apoptotic pathway in S. prolificans germlings via activation of metacaspases (Figure 4).
DISCUSSION
We hypothesized that TCR and 17AAG enhance the negligible activity of the ergosterol biosynthesis inhibitors PCZ and ICZ, to the point that they become fungicidal, and that this fungicidal activity is mediated through apoptosis in S. prolificans. The calcineurin pathway and Hsp90 are important for the survival of pathogenic fungi because they have central roles in various cellular processes, including morphogenetic transition and development of antifungal tolerance and resistance [7][16]. Inhibition of the calcineurin pathway and Hsp90 in combination with administration of conventional antifungal agents may have broad therapeutic potential in patients with fungal infections [16][25]. Owing to the immunosuppressive properties of calcineurin inhibitors and the role of Hsp90 in controlling the calcineurin pathway, clinical use of a combination of TCR or 17AAG with triazole for treatment of S. prolificans infection would ultimately require a novel antifungal agent that selectively targets fungal stress pathways without having collateral effects on human immune cells.
–
We found evidence of synergy of PCZ and ICZ with TCR and 17AAG in S. prolificans in vitro, which is consistent with data on other fungal species [12][16][18][21]. In addition, we used multiple markers of cell death to show that apoptosis is a mechanism of PCZ/ICZ- and TCR/17AAG-induced cell death. We corroborated the rate of apoptosis in S. prolificans germlings using assays for detection of phosphatidylserine (PS) by annexin V-FITC, ROS accumulation by DHR-123 staining and decreased mitochondrial membrane potential by Rh123, DNA damage by TUNEL staining, and activation of caspase-like activity by CaspACE FITC-VAD-FMK. In each of the assays, apoptosis was evident at PCZ, ICZ, TCR, and 17AAG concentrations (0.125-0.250 μg/ml) that were below the MIC of triazoles. Taken together, these data indicate that PCZ or ICZ combined with TCR or 17AAG at concentrations below the MIC causes apoptosis in S. prolificans germlings.
–
We found that induction of apoptosis and the fungicidal activity of PCZ and ICZ in combination with TCR or 17AAG correlated with increased plasma and mitochondrial membrane disruption, PS externalization, DNA fragmentation, and ROS accumulation in S. prolificans germlings (isolates 1 and 2) (Tables 1, 2 and S1, Figures 1-4). Calcineurin activity is known to contribute to the fungicidal effects of Hsp90 inhibitors. 17-AAG in particular induces apoptosis in colon carcinoma-derived cell lines [26], so determination of whether inhibition of Hsp90 can induce apoptotic cell death in fungi would be of interest. Dai et al. [27] demonstrated the role of Hsp90 in apoptosis in C. albicans and showed that inhibition of Hsp90 attenuated apoptosis by regulating the calcineurin pathway. Several fungi undergo apoptosis in response to antifungal treatment and various other stimuli [19]. Additional studies providing better understanding of fungal apoptotic pathways would promote the discovery of much-needed antifungal therapies.
–
Our results indicated that disruption of mitochondrial integrity by a combination of PCZ/ICZ with TCR or 17AAG induced apoptosis in S. prolificans. Our study in S. prolificans and studies in other fungi showed that 17AAG inhibits Hsp90, causing mitochondria-mediated apoptosis in rat histiocytomas [28]. Also, Shirazi and Kontoyiannis [18] showed that increased apoptosis after exposure to TCR was correlated with increased intracellular ROS accumulation in Mucorales. Furthermore, translocation of mitochondrial cyt c to the cytosol has led to binding of cyt c with apoptotic protease-activating factor to form a complex with caspase-9, resulting in caspase activation [23][24][29]. Release of cyt c requires an increase in mitochondrial membrane permeability during apoptosis [23]. As in Mucorales, our results in S. prolificans also demonstrated that, ROS formation, changes in ΔΨm, and cyt c release were associated with apoptosis [20][21][22]. Authors have also reported ROS-induced apoptosis in A. nidulans, Fusarium oxysporum, and C. albicans [30][31][32]. Sharon et al. [33] reported that apoptotic pathways in fungi seem to be mitochondrion-dependent, and can be powerful sources of superoxide radicals in cells undergoing miconazole and farnesol-induced apoptosis [34].
–
Authors have reported accumulating evidence that different stimuli induce different apoptotic pathways in yeasts and other fungi [35][36]. In mammals, apoptosis is regulated by activation of caspases, which cleave specific substrates and trigger apoptotic death [37]. Now it is evident that caspase-like proteolytic activity may exist not only in multicellular organisms but also unicellular organisms, such as fungi. In the current study, we observed caspase-like activity in S. prolificans germlings upon exposure to PCZ or ICZ with TCR or 17AAG. Further studies are needed to demonstrate how proteases contribute to apoptotic fungal death.
–
In conclusion, we have shown for the first time that co-administration of inhibitors of the ergosterol biosynthesis pathways with an inhibitor of calcineurin or Hsp90 induces apoptosis in the recalcitrant fungus S. prolificans. This fungicidal synergistic interaction requires further study, as it may be a useful therapeutic strategy for infections caused by pathogenic fungi for which treatment options are extremely limited.
MATERIALS AND METHODS
Drugs
PCZ stock (5 mg/ml; Merck & Co., Inc.) was prepared in distilled water. ICZ (5 mg/ml; Janssen Pharmaceuticals), TCR (1 mg/ml; Medisca), and 17AAG (Sigma) stocks were prepared in ethanol, and aliquots were stored at -20°C in the dark until use.
–
Isolates and growth conditions
Three clinical isolates of S. prolificans (S.p-071507 [isolate 1], 071826 [isolate 2], and 674802 [isolate 3]) were grown on freshly prepared Sabouraud dextrose agar plates. After 48 h of incubation at 37°C, spores were collected and washed twice in sterile phosphate-buffered saline (PBS). The spores were then counted using a hemocytometer and stored at 4°C in PBS.
–
Susceptibility testing
Broth microdilution was performed according to the Clinical and Laboratory Standards Institute method [38]. Briefly, two-fold serial PCZ and ICZ dilutions were prepared in flat-bottomed 96-well microtiter plates (100 µl/well) in the presence or absence of TCR or 17AAG (0.060-0.125 µg/ml). Drug-free wells were used as controls. Each well was inoculated with 100 µl of freshly isolated S. prolificans spores (2-3 days old; 1 × 104 spores/ml) suspended in RPMI medium. After 48 h of incubation at 37°C, the MICs of PCZ and ICZ were determined visually as the lowest drug concentrations resulting in complete growth inhibition. To determine the minimum fungicidal concentrations (MFC) of PCZ and ICZ, an aliquot (20 µl) from each well that exhibited 100% growth inhibition was plated onto YPD agar (1% yeast extract, 2% peptone, 2% dextrose and 2% agar) plates. After 24 h of incubation at 37°C, the MFC was recorded as the lowest drug concentration at which no growth was observed.
–
For all of the wells of the microtiter plates that corresponded to MICs, the sum of the fractional inhibitory concentrations (ΣFIC) was calculated for each well using the equation ΣFIC = FICA + FICB = (CA/MICA) + (CB/MICB), in which MICA and MICB are the MICs of drugs A and B alone, respectively, and CA and CB are the concentrations of the drugs in combination, respectively, in all of the wells corresponding to an MIC. Synergy was defined as a ΣFIC of up to 0.5. Indifference was defined as a ΣFIC of at least 0.5 but no more than 4.0. Antagonism was defined as a ΣFIC greater than 4.0.
–
Viability assay
S. prolificans germlings (isolates 1 and 2) treated with TCR or 17AAG (0.060-0.125 µg/ml) along with PCZ (0.06-0.50 µg/ml) or ICZ (0.06-0.25 µg/ml) for 3 h were stained with DiBAC (Molecular Probes) as described previously [21][22].
–
Annexin V-FITC–PI double staining of S. prolificans (isolates 1 and 2)
The apoptosis marker PS is located on the inner leaflet of the lipid bilayer of the cytoplasmic membrane and is translocated to the outer leaflet at the onset of apoptosis [39][40][41]. PS can be detected using staining with annexin V-FITC, which binds to it. Germlings treated with PCZ (0.06-0.50 µg/ml) or ICZ (0.06-0.25 µg/ml) in combination with TCR or 17AAG (0.060 and 0.125 µg/ml) were digested with a lysing enzyme mixture (0.25 mg/ml chitinase, 15 U of lyticase, and 20 mg/ml lysing enzyme; Sigma) for 3 h at 30°C. After digestion, S. prolificans protoplasts were stained with annexin V-FITC (BD Pharmingen) and PI at room temperature for 15 min and observed under a fluorescence microscope to assess the externalization of PS as described previously [39].
–
Detection of intracellular ROS accumulation and ΔΨmin S. prolificans germlings (isolates 1and 2)
ROS plays an important role as an early initiator of apoptosis in yeasts and other filamentous fungi [20][21][22]. The amount of ROS in S. prolificans germlings was measured using DHR-123 (Sigma) staining [20][21][22]. The mitochondrial membrane potential was assessed by staining with Rh-123 (Sigma), a fluorescent dye that diffuses in the matrix in response to electric potential as described [20][21][22]. Intracellular ROS levels and ΔΨm in S. prolificans germlings were measured after treatment with PCZ (0.060-0.50 µg/ml) or ICZ (0.060-0.25 µg/ml) in combination with TCR and 17 AAG (0.060 and 0.125 µg/ml) for 3 h at 37°C using a fluorimetric assay with DHR-123 and Rh-123 staining [20][21][22][42].
–
Measurement of DNA damage in S. prolificans (isolates 1 and 2)
DNA fragmentation, a characteristic of apoptosis, was detected in S. prolificans using a TUNEL assay. Germlings pretreated with PCZ (0.06-0.50 µg/ml) or ICZ (0.06-0.25 µg/ml) in combination with TCR or 17AAG (0.060 and 0.125 µg/ml) for 3 h at 37°C were fixed with 3.7% formaldehyde for 30 min on ice and digested using a lysing enzyme mixture. Enzyme-digested germlings were used to detect DNA fragmentation using a TUNEL assay as described by Madeo et al. [41]. The protoplasts were observed for fluorescence with excitation and emission wavelengths of 488 nm and 520 nm, respectively.
–
Detection of metacaspase activity using CaspACE FITC-VAD-FMK in S. prolificans germlings (isolates 1 and 2)
Active metacaspases in S. prolificans germlings were detected using CaspACE FITC-VAD-FMK (Promega) according to the manufacturer’s instructions [20][21][22]. Briefly, germlings pretreated with PCZ (0.06-0.50 µg/ml) or ICZ (0.06-0.25 µg/ml) in combination with TCR or 17AAG (0.060 and 0.125 µg/ml) for 3 h at 37°C were collected, washed in PBS, resuspended in 10 µM FITC-VAD-FMK, and incubated again for 2 h at 30°C. Apoptosis in the S. prolificans germlings was inhibited in the presence of the caspase inhibitor z-VAD-FMK (Sigma) at final concentrations of 40 µM. After incubation, germlings were washed twice in PBS and observed microscopically for fluorescence with excitation and emission settings of 488 nm and 520 nm, respectively.
–
Statistical Analysis
For all assays, three independent experiments were performed in triplicate. Comparisons of multiple treatment groups were performed by using two-way analysis of variance with post-hoc paired comparisons using Dunnett’s test. Calculations were made using the InStat software program (GraphPad Software). Two-tailed P values of less than 0.05 were considered statistically significant.
References
- I.B. Gosbell, M.L. Morris, J.H. Gallo, K.A. Weeks, S.A. Neville, A.H. Rogers, R.H. Andrews, and D.H. Ellis, "Clinical, pathologic and epidemiologic features of infection with Scedosporium prolificans: four cases and review", Clinical Microbiology and Infection, vol. 5, pp. 672-686, 1999. http://dx.doi.org/10.1111/j.1469-0691.1999.tb00513.x
- M. Cuenca-Estrella, B. Ruiz-Díez, J.V. Martínez-Suárez, A. Monzón, and J.L. Rodríguez-Tudela, "Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum", Journal of Antimicrobial Chemotherapy, vol. 43, pp. 149-151, 1999. http://dx.doi.org/10.1093/jac/43.1.149
- K.J. Cortez, E. Roilides, F. Quiroz-Telles, J. Meletiadis, C. Antachopoulos, T. Knudsen, W. Buchanan, J. Milanovich, D.A. Sutton, A. Fothergill, M.G. Rinaldi, Y.R. Shea, T. Zaoutis, S. Kottilil, and T.J. Walsh, "Infections Caused byScedosporiumspp", Clinical Microbiology Reviews, vol. 21, pp. 157-197, 2008. http://dx.doi.org/10.1128/CMR.00039-07
- G.A. Lamaris, G. Chamilos, R.E. Lewis, A. Safdar, I.I. Raad, and D.P. Kontoyiannis, "Scedosporium Infection in a Tertiary Care Cancer Center: A Review of 25 Cases from 1989-2006", Clinical Infectious Diseases, vol. 43, pp. 1580-1584, 2006. http://dx.doi.org/10.1086/509579
- T. Bader, K. Schröppel, S. Bentink, N. Agabian, G. Köhler, and J. Morschhäuser, "Role of Calcineurin in Stress Resistance, Morphogenesis, and Virulence of a Candida albicans Wild-Type Strain", Infection and Immunity, vol. 74, pp. 4366-4369, 2006. http://dx.doi.org/10.1128/IAI.00142-06
- L.E. Cowen, and S. Lindquist, "Hsp90 Potentiates the Rapid Evolution of New Traits: Drug Resistance in Diverse Fungi", Science, vol. 309, pp. 2185-2189, 2005. http://dx.doi.org/10.1126/science.1118370
- L.E. Cowen, "The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype", Nature Reviews Microbiology, vol. 6, pp. 187-198, 2008. http://dx.doi.org/10.1038/nrmicro1835
- J.L. Reedy, S.G. Filler, and J. Heitman, "Elucidating the Candida albicans calcineurin signaling cascade controlling stress response and virulence", Fungal Genetics and Biology, vol. 47, pp. 107-116, 2010. http://dx.doi.org/10.1016/j.fgb.2009.09.002
- W.J. Steinbach, R.A. Cramer, B.Z. Perfect, Y.G. Asfaw, T.C. Sauer, L.K. Najvar, W.R. Kirkpatrick, T.F. Patterson, D.K. Benjamin, J. Heitman, and J.R. Perfect, "Calcineurin Controls Growth, Morphology, and Pathogenicity in Aspergillus fumigatus", Eukaryotic Cell, vol. 5, pp. 1091-1103, 2006. http://dx.doi.org/10.1128/EC.00139-06
- S.K. Wandinger, K. Richter, and J. Buchner, "The Hsp90 Chaperone Machinery", Journal of Biological Chemistry, vol. 283, pp. 18473-18477, 2008. http://dx.doi.org/10.1074/jbc.R800007200
- B.D. Kahan, "Individuality: the barrier to optimal immunosuppression", Nature Reviews Immunology, vol. 3, pp. 831-838, 2003. http://dx.doi.org/10.1038/nri1204
- E. Dannaoui, J. Afeltra, J.F.G.M. Meis, and P.E. Verweij, "In Vitro Susceptibilities of Zygomycetes to Combinations of Antimicrobial Agents", Antimicrobial Agents and Chemotherapy, vol. 46, pp. 2708-2711, 2002. http://dx.doi.org/10.1128/AAC.46.8.2708-2711.2002
- D.P. Kontoyiannis, "Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species", Journal of Antimicrobial Chemotherapy, vol. 51, pp. 313-316, 2003. http://dx.doi.org/10.1093/jac/dkg090
- S. Narreddy, E. Manavathu, P.H. Chandrasekar, G.J. Alangaden, and S.G. Revankar, "In vitro interaction of posaconazole with calcineurin inhibitors and sirolimus against zygomycetes", Journal of Antimicrobial Chemotherapy, vol. 65, pp. 701-703, 2010. http://dx.doi.org/10.1093/jac/dkq020
- R.E. Lewis, R. Ben-Ami, L. Best, N. Albert, T.J. Walsh, and D.P. Kontoyiannis, "Tacrolimus Enhances the Potency of Posaconazole Against Rhizopus oryzae In Vitro and in an Experimental Model of Mucormycosis", Journal of Infectious Diseases, vol. 207, pp. 834-841, 2012. http://dx.doi.org/10.1093/infdis/jis767
- L.E. Cowen, S.D. Singh, J.R. Köhler, C. Collins, A.K. Zaas, W.A. Schell, H. Aziz, E. Mylonakis, J.R. Perfect, L. Whitesell, and S. Lindquist, "Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease", Proceedings of the National Academy of Sciences, vol. 106, pp. 2818-2823, 2009. http://dx.doi.org/10.1073/pnas.0813394106
- H. Lu, Z. Zhu, L. Dong, X. Jia, X. Sun, L. Yan, Y. Chai, Y. Jiang, and Y. Cao, "Lack of Trehalose Accelerates H2O2-Induced Candida albicans Apoptosis through Regulating Ca2+ Signaling Pathway and Caspase Activity", PLoS ONE, vol. 6, pp. e15808, 2011. http://dx.doi.org/10.1371/journal.pone.0015808
- F. Shirazi, and D.P. Kontoyiannis, "The Calcineurin Pathway Inhibitor Tacrolimus Enhances the In Vitro Activity of Azoles against Mucorales via Apoptosis", Eukaryotic Cell, vol. 12, pp. 1225-1234, 2013. http://dx.doi.org/10.1128/EC.00138-13
- M. Ramsdale, "Programmed cell death in pathogenic fungi", Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, vol. 1783, pp. 1369-1380, 2008. http://dx.doi.org/10.1016/j.bbamcr.2008.01.021
- E.M. Barbu, F. Shirazi, D.M. McGrath, N. Albert, R.L. Sidman, R. Pasqualini, W. Arap, and D.P. Kontoyiannis, "An Antimicrobial Peptidomimetic Induces Mucorales Cell Death through Mitochondria-Mediated Apoptosis", PLoS ONE, vol. 8, pp. e76981, 2013. http://dx.doi.org/10.1371/journal.pone.0076981
- F. Shirazi, and D.P. Kontoyiannis, "Mitochondrial Respiratory Pathways Inhibition in Rhizopus oryzae Potentiates Activity of Posaconazole and Itraconazole via Apoptosis", PLoS ONE, vol. 8, pp. e63393, 2013. http://dx.doi.org/10.1371/journal.pone.0063393
- F. Shirazi, M.A. Pontikos, T.J. Walsh, N. Albert, R.E. Lewis, and D.P. Kontoyiannis, "Hyperthermia Sensitizes Rhizopus oryzae to Posaconazole and Itraconazole Action through Apoptosis", Antimicrobial Agents and Chemotherapy, vol. 57, pp. 4360-4368, 2013. http://dx.doi.org/10.1128/AAC.00571-13
- X. Wu, W. Chang, A. Cheng, L. Sun, and H. Lou, "Plagiochin E, an antifungal active macrocyclic bis(bibenzyl), induced apoptosis in Candida albicans through a metacaspase-dependent apoptotic pathway", Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1800, pp. 439-447, 2010. http://dx.doi.org/10.1016/j.bbagen.2010.01.001
- J. Cho, and D.G. Lee, "The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis", Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1810, pp. 1246-1251, 2011. http://dx.doi.org/10.1016/j.bbagen.2011.08.011
- S.L. LaFayette, C. Collins, A.K. Zaas, W.A. Schell, M. Betancourt-Quiroz, A.A.L. Gunatilaka, J.R. Perfect, and L.E. Cowen, "PKC Signaling Regulates Drug Resistance of the Fungal Pathogen Candida albicans via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90", PLoS Pathogens, vol. 6, pp. e1001069, 2010. http://dx.doi.org/10.1371/journal.ppat.1001069
- I. Hostein, D. Robertson, F. DiStefano, P. Workman, and P.A. Clarke, "Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis.", Cancer research, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11358818
- B. Dai, Y. Wang, D. Li, Y. Xu, R. Liang, L. Zhao, Y. Cao, J. Jia, and Y. Jiang, "Hsp90 Is Involved in Apoptosis of Candida albicans by Regulating the Calcineurin-Caspase Apoptotic Pathway", PLoS ONE, vol. 7, pp. e45109, 2012. http://dx.doi.org/10.1371/journal.pone.0045109
- A. Taiyab, A.S. Sreedhar, and C.M. Rao, "Hsp90 inhibitors, GA and 17AAG, lead to ER stress-induced apoptosis in rat histiocytoma", Biochemical Pharmacology, vol. 78, pp. 142-152, 2009. http://dx.doi.org/10.1016/j.bcp.2009.04.001
- J. Cho, and D.G. Lee, "Oxidative stress by antimicrobial peptide pleurocidin triggers apoptosis in Candida albicans", Biochimie, vol. 93, pp. 1873-1879, 2011. http://dx.doi.org/10.1016/j.biochi.2011.07.011
- C.P. Semighini, J.M. Hornby, R. Dumitru, K.W. Nickerson, and S.D. Harris, "Farnesol‐induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi", Molecular Microbiology, vol. 59, pp. 753-764, 2005. http://dx.doi.org/10.1111/j.1365-2958.2005.04976.x
- C.P. Semighini, N. Murray, and S.D. Harris, "Inhibition ofFusarium graminearumgrowth and development by farnesol", FEMS Microbiology Letters, vol. 279, pp. 259-264, 2008. http://dx.doi.org/10.1111/j.1574-6968.2007.01042.x
- M.E. Shirtliff, B.P. Krom, R.A.M. Meijering, B.M. Peters, J. Zhu, M.A. Scheper, M.L. Harris, and M.A. Jabra-Rizk, "Farnesol-Induced Apoptosis in Candida albicans", Antimicrobial Agents and Chemotherapy, vol. 53, pp. 2392-2401, 2009. http://dx.doi.org/10.1128/AAC.01551-08
- A. Sharon, A. Finkelstein, N. Shlezinger, and I. Hatam, "Fungal apoptosis: function, genes and gene function", FEMS Microbiology Reviews, vol. 33, pp. 833-854, 2009. http://dx.doi.org/10.1111/j.1574-6976.2009.00180.x
- D. Kobayashi, K. Kondo, N. Uehara, S. Otokozawa, N. Tsuji, A. Yagihashi, and N. Watanabe, "Endogenous Reactive Oxygen Species Is an Important Mediator of Miconazole Antifungal Effect", Antimicrobial Agents and Chemotherapy, vol. 46, pp. 3113-3117, 2002. http://dx.doi.org/10.1128/AAC.46.10.3113-3117.2002
- D. Carmona-Gutierrez, T. Eisenberg, S. Büttner, C. Meisinger, G. Kroemer, and F. Madeo, "Apoptosis in yeast: triggers, pathways, subroutines", Cell Death & Differentiation, vol. 17, pp. 763-773, 2010. http://dx.doi.org/10.1038/cdd.2009.219
- A. Hamann, D. Brust, and H.D. Osiewacz, "Apoptosis pathways in fungal growth, development and ageing", Trends in Microbiology, vol. 16, pp. 276-283, 2008. http://dx.doi.org/10.1016/j.tim.2008.03.003
- M.O. Hengartner, "DNA destroyers", Nature, vol. 412, pp. 27-29, 2001. http://dx.doi.org/10.1038/35083663
- . Clinical and Laboratory Standard Institute, "Reference method for broth dilution Antifungal Susceptibility Testing of filamentous fungi. Approved standard. 2nd edition", M38-A2, CLSI, Wayne, PA, USA, 2008.
- F. Madeo, E. Fröhlich, and K. Fröhlich, "A Yeast Mutant Showing Diagnostic Markers of Early and Late Apoptosis", The Journal of Cell Biology, vol. 139, pp. 729-734, 1997. http://dx.doi.org/10.1083/jcb.139.3.729
- F. Madeo, E. Fröhlich, M. Ligr, M. Grey, S.J. Sigrist, D.H. Wolf, and K. Fröhlich, "Oxygen Stress: A Regulator of Apoptosis in Yeast", The Journal of Cell Biology, vol. 145, pp. 757-767, 1999. http://dx.doi.org/10.1083/jcb.145.4.757
- F. Madeo, E. Herker, C. Maldener, S. Wissing, S. Lächelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K. Fröhlich, "A Caspase-Related Protease Regulates Apoptosis in Yeast", Molecular Cell, vol. 9, pp. 911-917, 2002. http://dx.doi.org/10.1016/S1097-2765(02)00501-4
- X. Wu, A. Cheng, L. Sun, S. Sun, and H. Lou, "Plagiochin E, an antifungal bis(bibenzyl), exerts its antifungal activity through mitochondrial dysfunction-induced reactive oxygen species accumulation in Candida albicans", Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1790, pp. 770-777, 2009. http://dx.doi.org/10.1016/j.bbagen.2009.05.002
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
D.P.K. acknowledges the Frances King Black Endowed Professorship for Cancer Research. This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant P30CA016672.
COPYRIGHT
© 2014

Heat shock protein 90 and calcineurin pathway inhibitors enhance the efficacy of triazoles against Scedosporium prolificans via induction of apoptosis by Fazal Shirazi and Dimitrios P. Kontoyiannis is licensed under a Creative Commons Attribution 4.0 International License.