Back to article: Shepherding DNA ends: Rif1 protects telomeres and chromosome breaks
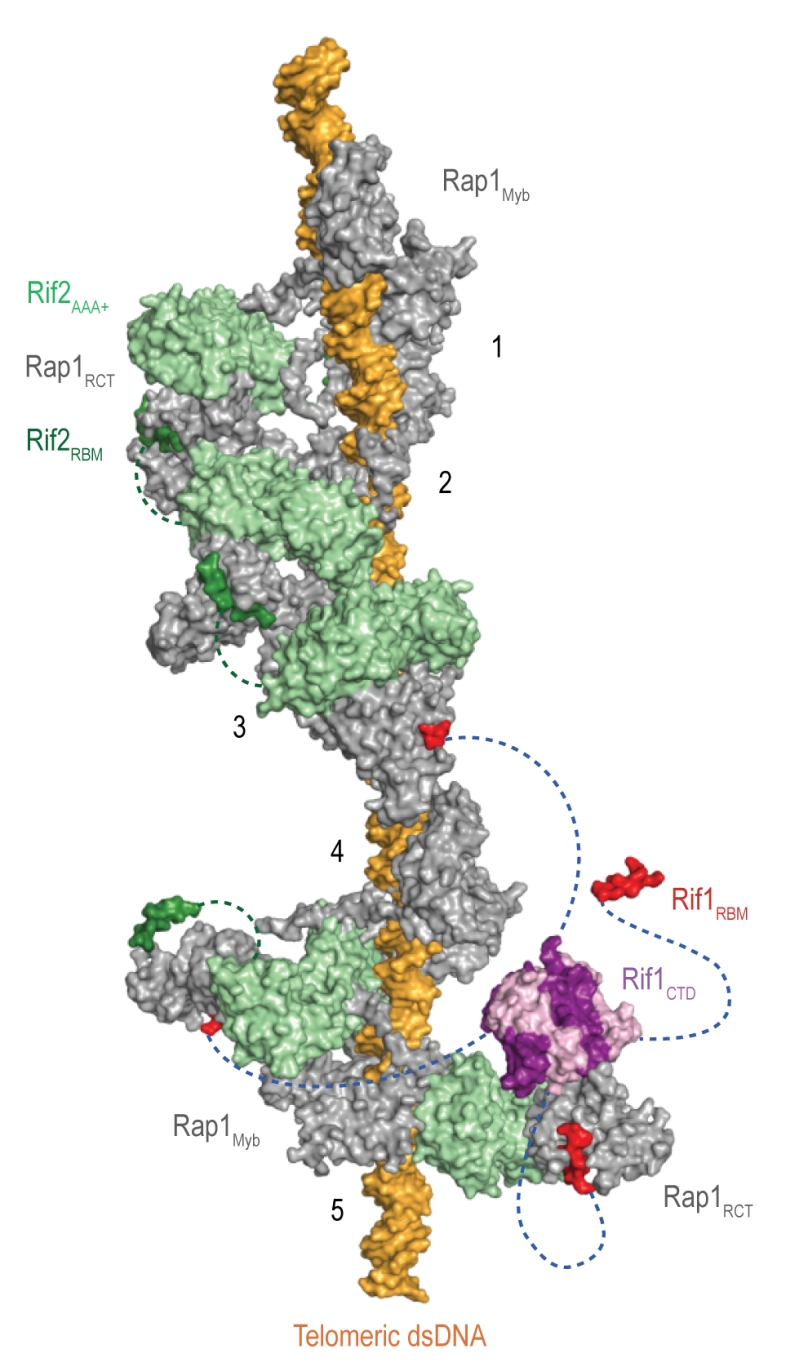
Figure 4: The Velcro-like protein network found at S. cerevisiae telomeres. Structural model illustrating the Rap1-Rif1-Rif2 interactions at dsDNA regions of budding yeast telomeres [22]. Rap1 (grey) engages DNA through its Myb domain (PDB: 1IGN [178]). Numbers 1 to 5 indicate Rap1-binding sites. Rif1 and Rif2 are recruited via the Rap1RCT domain (Rap1 C-terminal domain; linker regions not shown; PDB: 4BJ5 [22]; PDB: 3UKG [179]). In this example, each Rap1RCT is bound with Rif2 (PDB: 4BJ1 [22]) via the Rif2AAA+ domain (light green). The Rif2AAA+ domain is connected to the Rif2RBM (dark green), a second Rap1 interaction epitope with similar affinity for Rap1RCT [22]. A relatively short linker between Rif2AAA+ and Rif2RBM (green dotted line, maximal length of 42 Å) limits Rif2 to interlinking neighboring Rap1 molecules. At binding site 5, more complex Rap1-Rif1-Rif2 interactions are shown in exemplary fashion. The Rif1RBM (red, PDB: 4BJT) represents the major Rap1 interaction motif; the Rif1CTD (purple, PDB: 4BJS) plays an accessory role in Rap1 binding and serves as a tetramerization domain [22]. An extended flexible linker (blue dotted line) connects the RBM and CTD domains, allowing multimeric Rif1 to interlink up to four Rap1 units over long distances (maximal distance of 110 Å). The Rif1RBM and Rif2RBM bind Rap1RCT in a mutually exclusive manner at the same hydrophobic cleft. In addition, RBM-bound Rap1RCT can engage the Rif1CTD or Rif2AAA+ domains. These multipoint interactions between interconnected Rap1, Rif1, and Rif2 stabilize the telosome and have been likened to a molecular Velcro [22]. The Rap1BRCT and Rif1NTD domains have been omitted for clarity.
22. Shi T, Bunker RD, Mattarocci S, Ribeyre C, Faty M, Gut H, Scrima A, Rass U, Rubin SM, Shore D, Thoma NH (2013). Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell 153(6): 1340-1353. http://dx.doi.org/10.1016/j.cell.2013.05.007
178. Konig P, Giraldo R, Chapman L, Rhodes D (1996). The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell 85(1): 125-136. http://dx.doi.org/10.1016/S0092-8674(00)81088-0
179. Matot B, Le Bihan YV, Lescasse R, Perez J, Miron S, David G, Castaing B, Weber P, Raynal B, Zinn-Justin S, Gasparini S, Le Du MH (2012). The orientation of the C-terminal domain of the Saccharomyces cerevisiae Rap1 protein is determined by its binding to DNA.Nucleic Acids Res 40(7): 3197-3207. http://dx.doi.org/10.1093/nar/gkr1166