Reviews:
Microbial Cell, Vol. 11, No. 1, pp. 207 - 220; doi: 10.15698/mic2024.07.826
Neutralizing the threat: harnessing broadly neutralizing antibodies against HIV-1 for treatment and prevention
1 Department of Medicine, Division of Infectious Diseases, University of California, CA, 92697, Irvine, Irvine, USA.
Keywords: HIV1, bnAbs, prevention, treatment, viral reservoir.
Received originally: 17/10/2023 Received in revised form: 06/05/2024
Accepted: 15/05/2024
Published: 03/07/2024
Correspondence:
Johannes S Gach, Department of Medicine, Division of Infectious Diseases, University of California, Irvine, Irvine, CA 92697, USA; jgach@uci.edu
Conflict of interest statement: The authors declare that no competing interest exists.
Please cite this article as: Juan C Becerra, Lauren Hitchcock, Khoa Vu, Johannes S Gach (2024). Neutralizing the threat: harnessing broadly neutralizing antibodies against HIV-1 for treatment and prevention. Microbial Cell 11: 207-220. doi: 10.15698/mic2024.07.826
Abstract
Broadly neutralizing antibodies (bnAbs) targeting the human immunodeficiency virus-1 (HIV-1) have played a crucial role in elucidating and characterizing neutralization-sensitive sites on the HIV-1 envelope spike and in informing vaccine development. Continual advancements in identifying more potent bnAbs, along with their capacity to trigger antibody-mediated effector functions, coupled with modifications to extend their half-life, position them as promising candidates for both HIV-1 treatment and prevention. While current pharmacological interventions have made significant progress in managing HIV-1 infection and enhancing quality of life, no definitive cure or vaccines have been developed thus far. Standard treatments involve daily oral anti-retroviral therapy, which, despite its efficacy, can lead to notable long-term side effects. Recent clinical trial data have demonstrated encouraging therapeutic and preventive potential for bnAb therapies in both HIV-1-infected individuals and those without the infection. This review provides an overview of the advancements in HIV- 1-specific bnAbs and discusses the insights gathered from recent clinical trials regarding their application in treating and preventing HIV-1 infection.
INTRODUCTION
Antibody-mediated immunity is essential for the host defense system by eliminating disease-causing pathogens such as viruses or bacteria and preventing them from infecting human cells 1. Antibodies of the immunoglobulin G (IgG) isotype are the most abundant in humans and can recognize antigens with one or two Fab regions and interact with Fc gamma receptors (FcγRs) through the immunoglobulin Fc region 2, 3. IgG plays a vital role in the protection against human immunodeficiency virus-1 (HIV-1) infection, as demonstrated by numerous non-human primate studies 4, 5.
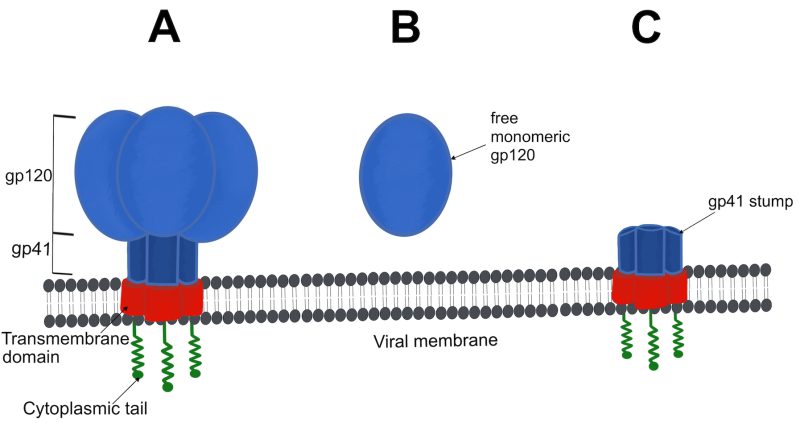
HIV-1 envelope spikes (Figure 1A), responsible for mediating the attachment and fusion between viral and host cell membranes 6, represent the primary targets of neutralizing immune responses in HIV-1-infected individuals 7, 8. However, HIV-1 has evolved evasive mechanisms by displaying major sequence variations on the envelope spike as well as a dense array of host-derived, immunologically “self”, N-linked glycans that efficiently shield the underlying protein from host antibody responses 9, 10. Moreover, HIV-1 virions typically exhibit a limited quantity of intact or functional envelope spikes on their surface, averaging seven to 14 spikes per viral particle 11, 12, 13. As a result, multivalent engagement with B cell receptors and, consequently, the B cell immune response against HIV-1 is limited 14, 15.
–
Furthermore, low spike numbers decrease the likelihood of bivalent antibody binding that might otherwise enhance neutralization 8, 16 or other antibody-mediated effector functions 17. In addition to the sparse number of functional envelope spikes, a high prevalence of non-functional forms of envelope, acting as decoys 18, 19, is present, which include soluble monomeric gp120 (Figure 1B) and fusion-incompetent gp41 stumps on the viral surface (Figure 1C). These non-functional forms of envelope succeed in diverting the immune responses toward immunodominant, non-neutralizing, epitopes rather than to the less accessible neutralizing epitopes 20. Accordingly, the first autologous neutralizing antibodies emerge relatively late following HIV-1 transmission (i.e., ~ twelve or more weeks) 21. These initial autologous strain-specific neutralizing antibodies are capable of blocking viral entry and preventing further host cell infection to some extent by (i) interfering with the engagement of CD4 receptors or co-receptors CCR5 and CXCR4, (ii) stabilizing the pre-fusion envelope spike to prevent membrane fusion, or (iii) accelerating envelope spike decay thereby applying selection pressure on cell-free HIV-1 virions 22.
Nevertheless, once HIV-1 enters and integrates into the host genome the error-prone viral reverse transcriptase, with a mutation rate of 5.4 x 10-5 per base per replication cycle 23, introduces random mutations into the envelope gene that can affect antibody-binding epitopes contributing neutralization-resistant variants that can continue infecting new cells 24. As a result, high mutation rates, combined with a short replication cycle and a tendency for recombination, induce quasispecies and generate viral diversity within an individual over the course of HIV-1 infection 25. A study that followed HIV-1 quasispecies generation in an infected couple revealed that, during the early stages, the distribution of virion varieties formed relatively tight phylogenetic clusters, becoming increasingly dispersed over time, with a corresponding increase in genetic diversity after one year 26. After three years, the viral sequences obtained from both partners gave rise to distinct phylogenetic clusters in the phylogenetic tree 26. Therefore, autologous neutralizing antibody responses are generally not associated with control of viremia in most HIV-1 infected individuals and lag behind viral escape variants 27.
While almost all infected individuals develop strain-specific neutralizing antibodies, specifically against autologous viruses, large clinical cohort studies revealed that approximately 10–30% of HIV-1 infections result in some level of serum neutralization breadth after several years of infection 28. In rare cases, around 1-2% of HIV-1-infected individuals, termed elite neutralizers, develop broadly neutralizing antibodies (bnAbs) 29, 30. The neutralization breadth of an antibody refers to its ability to recognize and neutralize different clades and strains of HIV-1 by targeting conserved sites on the functional envelope trimer 31. BnAbs can recognize and inactivate many different heterologous HIV-1 variants, while low-breadth autologous antibodies are more specific to the initially transmitted founder strains 31. The neutralization breadth of most bnAbs increases with the time of infection as they go through several rounds of somatic hypermutation 32. Nevertheless, the pathway to neutralization breadth also differs depending on the epitope being targeted 33.
BINDING EPITOPES OF HIV-1 bnAbs
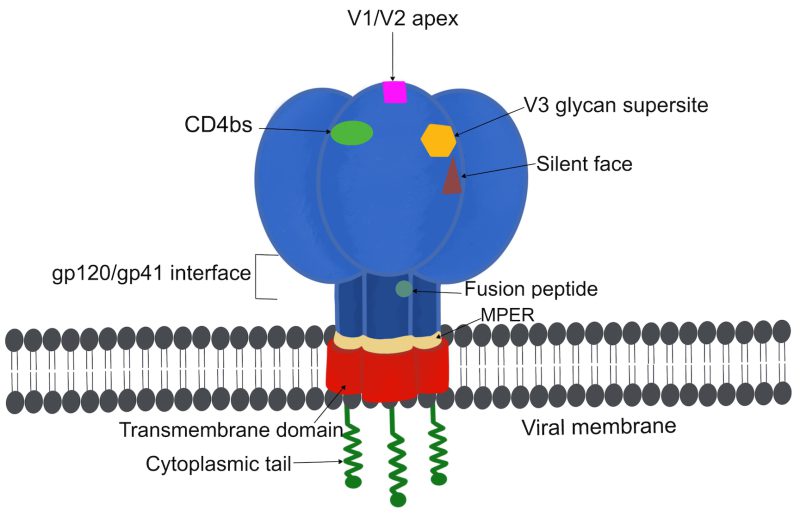
Recent advances in stabilizing and expressing a nearly complete form of the HIV-1 trimer vastly refined our understanding of the metastability of the trimer and its structures allowing for the detailed characterization of bnAb epitopes 34, 35. Sites of vulnerability (Figure 2) to bnAbs include the CD4 binding site (CD4bs), the V1/V2 loops at the trimer apex, the V3 glycan high mannose supersite, the silent face of gp120, the gp120-gp41 interface, the gp41 fusion peptide, and the membrane-proximal external region (MPER) 35, 36, 37, 38. Examples of bnAbs and their respective targets are shown in Table 1. Epitopes on the high-mannose patch are of particular interest for vaccine design considering they are frequently targeted by various classes of bnAbs, which do not require multiple rounds of maturation 39. A recent study that investigated an HIV-1 V2 loop-directed bnAb lineage reported that extensive antibody somatic hypermutation does not necessarily result in increased breadth and that many mutations do not impact or negatively affect breadth 40. While the majority of bnAbs can interact with both trimeric and monomeric envelope spikes, some bnAbs require intact quaternary structures that are believed to form only on the functional trimer 41, 42. In contrast, non-neutralizing antibodies typically bind to monomeric envelope subunits 43.
–
Table 1: Examples of broadly neutralizing HIV-1 envelope-specific antibodies and their respective binding sites.
|
Envelope Subunit |
Epitope |
Specific antibodies |
|---|---|---|
|
gp120 |
V1/V2 apex |
PG9 44; PG16 45; PGT141-145 46; CH01-04 47; PGDM1400 48; CAP256-VRC26.01-33 49; VRC38.01 50; PCT64 51 |
|
V3 glycan supersite |
2G12 52; PGT121 46; PGT128 46; PGT135 46;10-1074 53; PCDN-33A 54; BG18 55; DH270.6 56 |
|
|
CD4bs |
B12 57; VRC01 58; 3BNC117 59; 8ANC131 60; CH235.N6 61; PGV04 62; N49-P7 63; ACS101-103 64 |
|
|
Silent face |
||
|
gp120/gp41 interface |
||
|
gp41 |
Fusion peptide |
|
|
MPER |
CD4bs = CD4 binding site, MPER = membrane proximal external region.
EFFECTOR FUNCTIONS OF HIV-1 bnAbs
Recent data indicates that the principal mode of protection conferred by bnAbs involves the direct neutralization of virions 73, 74. However, bnAbs also elicit innate effector mechanisms via Fc-mediated interactions with FcγRs present on diverse immune cell types including natural killer (NK) cells, macrophages, and neutrophils 75, 76. This engagement triggers processes such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), both of which may substantially augment the overall protective efficacy 77, 78. Additionally, Fc-mediated complement-dependent effector functions, including complement-dependent cytotoxicity (CDC) or complement-dependent cellular phagocytosis (CDCP), have also been associated with the elimination of HIV-1 79. Both effector functions are usually initiated upon binding many molecules of IgG to a multivalent antigen or by high local antigen densities, which in turn allow the multimerization (i.e., hexamerization) of neighboring IgG-Fc domains 3, 79, 80. The hexameric structure forms an ideal platform for complement component 1q attachment and activation of the complement cascade that results in CDC or CDCP 81.
Clinical evidence of a protective role for Fc-mediated effector functions in HIV-1-infected individuals has been demonstrated in several studies 82, 83, 84, 85, 86, 87. For example, elevated levels of virus-specific ADCC in the patient serum/plasma have been correlated with slower disease progression 82, 83, 84. Fc-mediated effector functions also played an important role in controlling HIV-1 infection in elite controllers 85. Furthermore, ADCC was identified as an immune correlate of the modest protection observed in the RV144 vaccine trial 86, 87. Studies using passive immunization with bnAbs, and non-neutralizing antibodies in animal models, have provided valuable insights into the contribution of Fc-mediated effector functions to protective efficacy 88, 89, 90. While some experiments suggest a significant role for these functions, others report conflicting results. For instance, in non-human primate models, the protective effect of certain bnAbs was partially abrogated when Fc-receptor binding was eliminated, suggesting the involvement of Fc-mediated effector functions 91, 92. However, in other studies 73, 93, 94, the absence of evidence for a contribution of Fc-mediated effector function challenges this notion, raising questions about the variability in bnAb efficacy across different viral infection models.
Recent advances in mathematical modeling and HIV-1 dynamics have enabled quantification of the relative contribution of Fc-mediated effector functions to the antiviral activity of bnAbs, particularly in contexts where established infections need to be treated 84, 93. These models suggest that Fc-mediated effector functions can account for a substantial portion (around 20-45%) of bnAb activity. On the other hand, investigations into engineered bnAb variants with enhanced FcγR binding aimed at boosting ADCC activity have brought about mixed results 93, 95, calling attention to the complex role of effector functions in modulating therapeutic outcomes.
Therefore, having an understanding of the interplay between Fab-mediated neutralization and Fc-mediated effector functions, as well as the factors influencing their effectiveness, is crucial for optimizing antibody-based strategies for HIV-1 prevention and therapeutic intervention. One of the ways by which Fc-mediated effector functions confer protection against viral infections, thereby contributing to overall efficacy is by targeting infected cells for elimination as seen in a recent clinical trial 96. Cells infected with HIV-1 exhibit increased vulnerability to elimination compared to cell-free HIV-1 virions through ADCC and ADCP, owing to the diminished abundance of envelope spikes on the surface of these virions 97. The presence of fewer spikes makes cell-free HIV-1 virions less favorable targets for immune-mediated clearance, even in the presence of complement 98. Further research into the differential abilities of bnAbs to recruit effector functions in vivo will be essential for advancing antibody-based HIV-1 therapies.
CHALLENGES OF ELICITING bnAbs
HIV-1-specific bnAbs have been evaluated in various populations and clinical scenarios 99. Evidence that bnAbs play a key role in controlling new and pre-existing HIV-1 infections supports the idea that a vaccine capable of eliciting bnAbs could provide the immune system with the head start necessary to prevent HIV-1 infection 22. Despite recent advances in vaccine design, such as soluble envelope trimer engineering, eliciting bnAbs against HIV-1 remains particularly challenging 34 given that most targets displayed on the viral envelope spike require prolonged antibody maturation pathways to be generated and effective 100. Besides extensive somatic hypermutation, bnAbs feature other unusual characteristics such as long complementary determining regions (CDRs), polyreactivity, and a high prevalence of rare precursor genes 101. To address these challenges, novel strategies such as targeting germline precursors of bnAbs have been implemented for HIV-1 vaccine design 102, 103. A recent phase 1 clinical trial with a germline-targeting priming vaccine candidate (IAVI G001) reported the presence of VRC01-class bnAb precursors in 97% of the vaccine recipients 104. The key to the apparent success appears to be the targeting of germinal B-cells with a nanoparticle carrying 60 copies of an engineered antigen (eOD-GT8 60mer) resembling the inferred immunogenic precursor target of VRC01 recognized by immature B cells, and a subsequent inoculation with a more refined version of the antigen of interest. This guided immune response resulted in the production both VRC01-class B-cells precursors and memory B cells 104. These encouraging results establish a clinical proof of concept for germline-targeting vaccine priming and support the development of boosting regimens to further enhance bnAb maturation. Currently, two clinical trials (NCT05001373 and NCT05414786) are being conducted to assess the safety and immunogenicity of mRNA-based vaccines based on the eOD-GT8 60mer 105.
HIV-1 bnAbs FOR TREATMENT
Antiretroviral therapy (ART) has effectively lowered HIV-1-associated morbidity and mortality rates by suppressing viral replication 106 and demonstrated remarkable efficacy in prevention 107. However, ART does not eradicate established HIV-1 infections, thus highlighting the need for a life-long treatment to prevent HIV-1 reactivation from long-lived viral reservoirs 108, 109, 110. Most HIV-1-infected individuals who discontinue ART typically exhibit a viral rebound within weeks even including those who initiated ART early during acute infection with long-term suppression of plasma viremia 111. Due to barriers to universal ART uptake including stigma, adverse effects, emerging drug resistance and reluctant to adhere to lifelong ART medication, novel preventive and therapeutic interventions are highly desirable 112, 113. At a minimum, alternative intervention characteristics should include durable viral suppression below viral transmission levels, prevention of disease progression, low susceptibility to drug resistance, and being generally safe and reasonably tolerated 111.
To date, HIV-1-specific bnAbs are being thoroughly investigated as potential ART alternatives because they directly target specific viral epitopes and potentially harness host immune responses 114. While first-generation bnAbs were generally safe when administered in the context of ongoing viremia or during ART interruption, they had limited potency and limited antiviral activity after multiple high-dose infusions 115, 116. In contrast, second-generation bnAbs revealed enhanced potency requiring less frequent administrations to maintain serum concentrations at acceptable target levels 117. Multiple potent second-generation bnAbs against HIV-1 have been and are currently being assessed in clinical trials for HIV-1 treatment (Table 2). As of now, single, combination, and repeated administrations of these second-generation bnAbs have been generally well tolerated with rare adverse events 111.
Table 2: Examples of completed and active clinical trials using second-generation HIV-1bnAbs for the treatment of HIV-1.
|
CLINICAL TRIAL IDENTIFIER |
BROADLY NEUTRALIZING ANTIBODIES |
ENVELOPE BINDING SITE |
|---|---|---|
|
COMPLETED |
||
|
NCT02960581 |
PGT121 |
V3 glycan |
|
NCT03205917 |
PGDM1400 PGT121 VRC07-523LS |
V1/V2 loop V3 glycan CD4bs |
|
NCT02018510 |
3BNC117 |
CD4bs |
|
NCT02446847 |
3BNC117 |
CD4bs |
|
NCT02588586 |
3BNC117 |
CD4bs |
|
NCT02825797 |
3BNC117 10-1074 |
CD4bs V3 glycan supersite |
|
NCT03526848 |
3BNC117 10-1074 |
CD4bs V3 glycan supersite |
|
NCT02511990 |
10-1074 |
V3 loop |
|
NCT03707977 |
VRC01LS 10-1074 |
CD4bs V3 glycan supersite |
|
NCT02664415 |
VRC01 |
CD4bs |
|
NCT01950325 |
VRC01 |
CD4bs |
|
NCT02840474 |
VRC01LS VRC07-523LS |
CD4bs CD4bs |
|
NCT03254277 |
3BNC117-LS |
CD4bs |
|
NCT04250636 |
3BNC117-LS 10-1074-LS |
CD4bs V3 glycan supersite |
|
NCT03875209 |
10E8.4 |
MPER |
|
NCT02579083 |
VRC01-N |
CD4bs |
|
NCT03015181 |
VRC01-523LS |
CD4bs |
|
NCT03837756 |
3BNC117 10-1074-LS |
CD4bs V3 glycan supersite |
|
NCT03041012 |
3BNC117 |
CD4bs |
|
ACTIVE |
||
|
NCT04871113 |
GSK3810109A |
CD4bs |
|
NCT04319367 |
10-1074-LS |
V3 glycan supersite |
|
NCT05300035 |
10-1074-LS |
V3 glycan supersite |
|
NCT03374202 |
AAV-VRC07 |
CD4bs |
|
NCT04357821 |
VRC07-523LS 10-1074 |
CD4bs V3 glycan supersite |
|
NCT04173819 |
3BNC117-LS-J 10-1074-LS-J |
CD4bs V3 glycan supersite |
|
NCT04983030 |
PGDM1400 PGT121 VRC07-523LS |
V1/V2 loop V3 glycan CD4bs |
|
NCT05281510 |
VRC07-523LS CAP256V2LS |
CD4 V1/V2 loop |
|
NCT05719441 |
VRC07-523LS PGT121.414.LS |
CD4bs V3 glycan |
Clinical data from prevention trials (Table 2) consistently demonstrate that bnAbs extend viral suppression, regardless of ART. However, their effectiveness depends on virus sensitivity and therapeutic antibody levels. In recent trials, a single infusion of bnAb 3BNC117 (NCT02018510) reduced HIV-1 viremia for up to 28 days, but with the emergence of resistant strains 59. BnAb VRC01 (NCT01950325) showed reduced viremia, though some participants had minimal response due to resistant virus 118. Single doses of bnAb 10-1074 (NCT02511990) and PGT121 (NCT02960581) led to the rapid emergence of resistant viruses, despite their potency 119, 120. In contrast, multiple bnAb doses, as shown in a phase 2a trial (NCT02446847), may prolong suppression, with notable delays in viral rebound compared to historical controls 121. These findings underscore the potential limitations of single-dose bnAb regimens and highlight the importance of exploring multiple dosing strategies to achieve sustained viral suppression in HIV-1-infected individuals.
Combining bnAbs targeting various HIV-1 neutralizing epitopes has been trialed to combat resistance. Recent phase 1 trials showed that pairing 3BNC117 and 10-1074 increased viral suppression with fewer escape mutants. One trial (NCT02825797) reported 15 weeks of suppression without mutants after three doses at weeks 0, 3, and 6 122. Another trial with an eight-dose regimen over 28 weeks achieved complete viral suppression for 43 weeks post-ART interruption or 15 weeks post-infusion, without escape mutants 123. However, due to HIV-1 diversity, this combination may only benefit approximately 50% of clade B-infected individuals sensitive to both antibodies 122. Testing a triple bnAb formulation (NCT03205917) consisting of PGDM1400, PGT121, and VRC07-523LS showed promising coverage; however, viral rebound occurred within a median of 20 days post-infusion for two (i.e., PGDM1400 and PGT121) out of the three antibodies 124, indicating the need for further refinement in antibody selection for sustained effectiveness.
Potential improvements in antibody selection may involve conducting genotypic and phenotypic analysis of plasma virus samples 125, akin to the Zurich Primary HIV Infection Study 126, to identify candidates responsive to specific bnAbs. Additionally, implementing computational studies capable of predicting viral rebound and delineating HIV-1 escape pathways from other bnAbs, for which therapy trials are lacking, could inform the design of optimal treatments 127, 128.
Furthermore, antibody Fc domain engineering methods to significantly improve the half-life of existing bnAbs have been successfully implemented in recent clinical trials 129, 122, 130. The half-life of circulating IgGs is regulated through Fc binding to the neonatal Fc receptors protecting them from lysosomal degradation 131, 132. To increase the half-life of an IgG molecule, a serine and leucine double mutation can be introduced at positions M428L and N434S in the Fc domain. The double mutation not only increases the serum half-life and the persistence in genital tissues but also retains neutralizing antibody levels for longer time133, 134. Such changes in LS-antibodies are expected to be translated into administering bnAbs treatments at 3–6-month intervals, which could be a promising alternative to ART regimens, especially in geographically isolated locations. In summary, although the clinical trials utilizing bnAbs discussed above have shown promise, further fine-tuning in the selection of bnAbs and their treatment schedules is necessary to optimize prevention effectiveness in both ART-treated and untreated individuals.
HIV-1 bnAbs AND VIRAL RESERVOIRS
The HIV-1 viral reservoir consists of latently infected cells such as resting CD4+ T cells and other long-lived cells that harbor integrated HIV-1 DNA within their genome, allowing the virus to persist in the body for extended periods 135. The presence of these reservoirs is a critical obstacle to viral eradication. The reservoirs are usually established within the first few days of infection and enable viral rebound after ART interruption despite generally efficient suppression of viral replication by ART 136. However, early ART initiation during acute infection may limit the size and diversity of HIV-1 reservoirs compared to late ART initiation during chronic infection 112, 136, 137.
In the search for an eradication strategy, various approaches for reducing and eliminating viral reservoirs have been investigated, including, but not limited, to the “shock and kill” strategy, which aims to reactivate dormant infected cells for immune clearance 138, 139; gene editing techniques like CRISPR/Cas9 to directly target and disrupt viral DNA 140, 141; immune-based therapies such as therapeutic vaccines and immunomodulatory agents to enhance immune response 142, 143, 144; development of novel antiretroviral drugs with enhanced potency 145, 146; and exploration of combination approaches integrating multiple strategies for more effective reservoir targeting 147, 148. An antibody-based strategy for eliminating viral reservoirs, employing bnAbs to target and eradicate HIV-infected cells, has also been explored 149. While earlier stage 1 clinical trials showed limited success 118, 150,151 recent preclinical studies and early clinical trials have demonstrated the potential to diminish viral reservoir and delay viral rebound 96, 152, 153 . For example, a recent phase 1b/2a study (NCT03041012) assessed whether early intervention with bnAb 3BNC117 followed by a histone deacetylase inhibitor (romidepsin) shortly after ART initiation could alter the course of HIV-1 infection. The authors found that early intervention with 3BNC117 at ART initiation enhanced elimination of plasma viruses and infected cells, boosted HIV-1-specific CD8+ immunity, and was associated with sustained ART-free virologic control among individuals with 3BNC117-sensitive virus 152. These findings lend evidence to interventions administered at the time of ART initiation as a strategy to limit HIV-1 persistence. Another recent phase 1b study examined the impact of multiple infusions of 3BNC117 and 10-1074 in individuals chronically infected with HIV on sustained viral suppression without ART and reservoir size when administered independently 96. Analysis of the reservoir after six months of therapy indicated alterations in the size and makeup of the intact proviral reservoir with no observed reduction in the defective reservoir.
Understanding the differences in viral reservoirs and susceptibility to bnAbs between individuals starting ART early versus those with chronic infection is essential for designing clinical trials and implementing HIV-1 cure strategies focused on reducing viral reservoirs 136. For example, children living with HIV-1 and receiving ART from birth are particularly suitable for bnAb treatment due to their limited viral resistance and smaller viral reservoir size 154 . Moreover, they offer an opportunity for ART-sparing strategies, avoiding long-term toxicities and adherence issues. A bnAb treatment study in Botswana (NCT03707977) achieved sustained HIV-1 suppression in children with favorable reservoir characteristics, including a smaller initial proviral reservoir and sensitivity to bnAb neutralization 129 . Despite these promising results, challenges remain, such as understanding the long-term effects of antibody infusions in children, the establishment of long-lived viral reservoirs, and their susceptibility to bnAbs. To tackle the latter, various combinations of bnAbs with potent latency-reversing agents or other immunomodulatory approaches, such as toll-like receptor agonists, cytokines, checkpoint blockade inhibitors, or therapeutic vaccines, are currently under investigation 99.
Although promising, there are lingering questions about the effectiveness and accessibility of bnAbs in tissues compared to the bloodstream, as well as concerns about viral escape mutations and the selection pressures influencing viral rebound in the presence of non-suppressive bnAb therapy. Nonetheless, therapies based on bnAbs offer a promising avenue for researching an HIV-1 cure, and are central to further exploration in current studies.
ROLE OF bnAbs FOR PREVENTION
Whether bnAbs can be used to prevent HIV-1 acquisition has been recently assessed in two randomized phase 2b efficacy clinical trials conducted in the Americas and Europe (HVTN 704/HPTN 085; NCT02716675) as well as in sub-Saharan Africa (HVTN 703/HPTN 081, NCT02568215) 155. Both proof of concept studies used the CD4bs-specific antibody VRC01 administered either at a low dose of 10 mg/kg or at a high dose of 30 mg/kg. Both trials showed that VRC01 was not able to prevent HIV-1 acquisition more efficiently than placebo. Out of 2699 participants in HVTN 704/HPTN 085, 32 infections occurred in the low dose group, 28 infections in the high dose group, and 38 infections in the placebo group. The estimated prevention efficacy of the pooled groups versus placebo was 26.5%.
Among the 1924 participants in HVTN 703/HPTN 081, 28 HIV-1 infections occurred in the low-dose group, 19 in the high-dose group, and 29 in the placebo group. Similarly, the estimated prevention efficacy was non-significant at 8.8%. Although VRC01 did not prevent overall HIV-1 acquisition more effectively than placebo a pooled data analysis looking at the incidence of infection with VRC01-sensitive isolates revealed an estimated prevention efficacy of over 75% suggesting that bnAb prophylaxis could be effective. Recently, a more potent and half-life enhanced bnAb (VRC07-523LS) was also tested in a phase 1 study (NCT03015181) and deemed safe for clinical use 130. It will be of interest to see whether this antibody reveals a better prevention efficacy compared to the previous trials.
More clinical studies are beginning to focus on bnAb combination regimens as an alternative to single regimens like VRC01 (Table 3). Currently, a double regimen including the two bnAbs CAP256V2LS and VRC07-523LS was tested for safety and pharmacokinetics. The phase 1 clinical trial conducted in South Africa with 42 HIV-negative participants (CAPRISA 012B) reported no serious adverse events or dose-limiting toxicities 117. Another phase 1 clinical trial is looking at the safety and pharmacokinetics of the bnAb combination 3BNC117 and 10-1074 in 25 HIV-1 negative participants for prophylactic use against HIV-1 (NCT02824536) 156.
Table 3: Examples of completed and active clinical trials using second-generation HIV-1bnAbs for the prevention of HIV-1.
|
CLINICAL TRIAL IDENTIFIER |
BROADLY NEUTRALIZING ANTIBODIES |
ENVELOPE BINDING SITE |
|---|---|---|
|
COMPLETED |
||
|
NCT02568215 |
VRC01 |
CD4bs |
|
NCT02716675 |
VRC01 |
CD4bs |
|
NCT02824536 |
3BNC117 10-1074 |
CD4bs, V3 glycan supersite |
|
ACTIVE |
||
|
NCT04408963 |
CAP256V2LS |
V1/V2 Loops |
Aside from infusions, a recent phase 1 clinical study (NCT02579083) described the use of MB66, a multipurpose prevention technology vaginal film product, in combination with bnAb VRC01-N against HIV-1 and HSV8-N against HSV-1 and 2 157 . MB66 was well tolerated when administered over seven consecutive days. The study observed peak antibody levels in vaginal secretions one-hour post-film administration, which remained significantly elevated through 24 hours. Vaginal samples collected after 24 hours significantly neutralized both HIV-1 and HSV-2 ex vivo.
CONCLUSION
The administration of HIV-1 envelope-specific bnAbs in humans has shown both safety and efficacy in reducing viremia. Moreover, bnAbs maintain short-term viral suppression of antibody-sensitive HIV-1 variants in the absence of ART. While both single-dose and multi-dose regimens are viable, multi-dose regimens have demonstrated prolonged HIV-1 suppression. Notably, individuals infected with HIV-1 who exhibit high sensitivity to specific bnAbs experience superior and longer-lasting viral suppression compared to those with lower sensitivity. Although combinatorial therapy with multiple bnAbs has partially addressed sensitivity issues, genotyping and phenotyping of locally circulating viruses will be essential for optimizing therapy outcomes. Enhanced bnAb combination therapies with extended intervals between treatments could offer patients greater flexibility without the need for daily oral medications like ART.
In the context of passive HIV-1 vaccination, HIV-1 envelope-specific bnAbs have been tested in high-risk uninfected populations, yielding limited protection. Initial clinical trials using a single bnAb regimen, such as VRC01, failed to achieve statistically significant protection compared to unvaccinated controls. Therefore, further studies employing combinatorial antibody regimens and improved antibody candidates, particularly those with extended half-lives, are necessary to explore the prophylactic potential of HIV-1-specific bnAbs, especially in high-risk groups such as men and transgender individuals who have sex with men.
The persistence of viral reservoirs poses a challenge to HIV eradication efforts despite effective ART. Various strategies are being explored, including antibody-based approaches using bnAbs to target and eliminate HIV-infected cells with recent studies showing promise in reducing the viral reservoir and delaying viral rebound. However, challenges remain in understanding differences in viral reservoirs and bnAb susceptibility, tissue accessibility, viral escape mutations, and rebound under non-suppressive bnAb therapy. Despite these challenges, bnAb-based therapies offer hope for significant advances towards eliminating HIV-1 infections.
ACKNOWLEDGMENTS
The authors thank Valerie Gach for crafting Figure 1 and Figure 2 with Procreate (Version 5.3.6).
COPYRIGHT
© 2024

Neutralizing the threat: harnessing broadly neutralizing antibodies against HIV-1 for treatment and prevention by Becerra et al. is licensed under a Creative Commons Attribution 4.0 International License.