Editorial Policies
- Peer review procedures
- Publication charges
- Copyright and license policies
- ICMJE best practice standards
- Publishing ethics
- Authorship
- Conflict of interest
- Informed consent
- Materials and data distribution
- Guidelines for specific study designs
- Human and animal research
- Standards
- Preparation and submission of manuscripts
- Press coverage of accepted papers
- Scientific misconduct and retraction
Peer Review Procedures
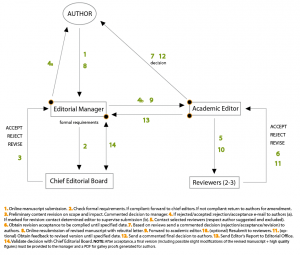
Any manuscript submitted to Microbial Cell is treated as a privileged document entrusted to academic editors and reviewers for evaluation. Microbial Cell is aware that such work is of great value for and proprietary to the author(s) and thus respects the need for confidentiality. That is why academic editors and reviewers need to adhereto confidentiality terms before obtaing access to the authors’ work. The manuscript is accurately reviewed, which includes (i) a formal check to ensure compliance with the journal’s policies and (ii) an academic evaluation by a member of the editorial board. This Handling Editor obtains the opinion of at least two external Reviewers and decides to (a) reject it, (b) accept it, or (c) request a minor or major revision. In the case of a revision, the Authors have up to three months (depending on the revision’s extent) to address the Reviewers’ and Handling Editor’s requests and concerns. However, the authors may contact the journal for an extension. After resubmission of the revised manuscript, the Handling Editor makes her/his final decision (rejected or accepted). In general, the peer review policy of Microbial Cell is single blinded (the reviewers are anonymous to the author but the reviewers know who the authors are). Microbial Cell also encourages its Editors to do all they can to ensure timely processing of manuscripts with the resources available to them. If Editors intend to publish a manuscript, they must do so in a timely manner and – if the case requires – negotiate with the authors any planned delays. If Microbial Cell has no intention of proceeding with a manuscript, the Handling Editor will reject the manuscript as soon as possible to allow authors to submit to a different journal. >> Back to top
Publication charges
The financing of Microbial Cell’s policy to provide open access to all published articles relies on a business model that charges a publication fee/article processing charges (APCs) to the authors or research sponsors for each article published to partly compensate the journal’s expenses such as those arising from peer review, journal production and archiving, or online hosting. Upon acceptance for publication, the author(s) will be issued with an invoice for payment of a publication fee. Published papers appear electronically and are freely available from Microbial Cell’s website. Authors may also use their published articles (including PDFs) on their own or institution’s website.
The publication fees applied for accepted articles are listed below (in EURO excluding applicable VAT; please note that fluctuations in foreign exchange rates may alter the payable fee in a different currency from invoice to invoice). All articles (including Editorials, Viewpoints and Meeting Reports) are subject to a fee unless they have been invited by the Editorial Board (see also article types).
| Article Type | Publication fee |
| Research Article | 1880 |
| Research Report | 1880 |
| Review | 1880 |
| Commentary articles | 975 |
| Invited articles | None |
Members of the Editorial Board are entitled to a 15% discount. Under certain circumstances or for submissions eligible for our Microbial Cell DevResearch program, the publication fee may be partially or completely waived. Please note that payments are accepted in EURO (€) only: upon payment in a differnt currency, handling fees will be applied for wire transfers (€20 excluding applicable VAT) and checks (€60 excluding applicable VAT) to cover additional administrative and bank charges. Unfortunately, payments via PayPal or credit card are currently not accepted. >> Back to top
Copyright and License Policies
Microbial Cell applies the Creative Commons Attribution (CC BY) license to all works we publish (read the human-readable summary or the full license legal code). Under the CC BY, authors retain ownership of the copyright for their article, but authors allow anyone to download, reuse, reprint, modify, distribute, and/or copy articles in Microbial Cell journal, so long as the original authors and source are cited. No permission is required from the authors or the publishers.
In most cases, appropriate attribution can be provided by simply citing the original article. If the item you plan to reuse is not part of a published article (e.g. a featured issue image), then please indicate the originator of the work, and the volume, issue, and date of the journal in which the item appeared. For any reuse or redistribution of a work, you must also make clear the license terms under which the work was published. This broad license was developed to facilitate open access to, and free use of, original works of all types. Applying this standard license to your own work will ensure your right to make your work freely and openly available. Learn more about our commitment to open access. If you have any license-related questions, please contact us.
Upon acceptance of a manuscript, the author(s) will be asked to fill and return a License to Publish form in order that the article can be published. Thereby, the author(s) retain(s) the publishing rights of his/her/their article without restrictions and without a time limit. >> Back to top
ICMJE best practice standards
Microbial Cell requires its Authors and Editors to follow the recommendations by the International Committee of Medical Journal Editors (ICMJE), which reviews best practice and ethical standards in the conduct and reporting of research and other material published in medical journals. It is Microbial Cell’s conviction that following these recommendations supports the accurate, clear, reproducible, and unbiased creation and distribution of scientific journal articles. Microbial Cell is officially indexed in the ICMJE journal list as a publication that follows these standards; please download or review these recommendations here.
These recommendations establish conduct guidelines at several levels and may be found throughout the description of our different policies, including but not limited to the definition of authorship for articles published in Microbial Cell, the necessity to report conflicts of interest for authors, reviewers and editors, the confidentiality to which any work submitted to Microbial Cell is subjected during its assessment, peer-review and production phases, the timeliness and integrity of editorial decisions, all ethical aspects with respect to research involving humans and animals (including informed consent of patients), editorial procedures concerning corrections and retractions, copyright issues, the description of applicable fees, practices in relation to sponsorships and advertising, issues about link stability and content preservation, principles applied on interactions with the media, requirement of registration of clinical trials in a public trials registry, or manuscript preparation and submission. >> Back to top
Publishing ethics
As a communication platform that aims at delivering reliable information to researchers and the public, Microbial Cell is aware of the importance – not only to apply a peer-review policy – but also to follow an ethical code in the act of scientific publishing. Microbial Cell interprets these ethics as a joint and agreed effort from all parties involved in the process: the author(s), the editor(s), the reviewer(s), and the journal as the hub between them.
Microbial Cell requires from the author(s) of a submitted manuscript to present their work with the expected integrity that warrants a global scientific advancement. Above all, Microbial Cell only publishes original work. With the submission of a manuscript, the authors confirm that the submitted paper is original, has not already been published in whole or in substantial part elsewhere, and does not infringe the copyright or other rights of any other person(s). Furthermore, they certify that it is not currently under consideration by any other journal. Any act of plagiarism will lead to the rejection of submitted material or – if already published – to the retraction of the corresponding paper. In addition, Microbial Cell has different editorial policies regarding for instance material and data access, authorship, specific study designs, human and animal research, or conflicts of interest. Should the author(s) discover a fundamental error in their work after publication, they are requested to contact Microbial Cell in order to issue a correction note (corrigendum). Thus, a corrigendum is the notification of an important error made by the author(s) that affects the scientific integrity of the article. It is also possible that an error is made by the journal upon production of the paper (erratum). Thus, an erratum is the notification of an important error made by the journal that affects the scientific integrity of the article. If an error is discovered (by the author(s), the journal, or a reader) so fundamental that it makes a paper obsolete, it will have to be retracted for the sake of scientific correctness. Corrigenda, errata and retractions are published as corresponding notifications in a subsequent issue of the article in question.
Microbial Cell requires from its Editors to make a fair publication decision when handling a manuscript assigned to them. Before an Editor accepts to act as the Handling Editor of a given manuscript she/he has to disclose any conflict of interest. In addition, upon acceptance to become a member of Microbial Cell’s Editorial Board, an Editor-to-be must explicitly accept the journal’s Confidentiality Agreement. Similarly, any referee contacted to review a submitted manuscript is prompted to deliver a fair comment in a timely manner. Before receiving a full manuscript, a referee must disclose any conflict of interest and subscribe to a confidentiality code stating that privileged information or ideas obtained through peer review must be kept confidential and not used for competitive gain.
Beyond these ethical standards, Microbial Cell requires its authors and editors to follow the recommendations by the International Committee of Medical Journal Editors (ICMJE), which reviews best practice and ethical standards in the conduct and reporting of research and other material published in medical journals. >> Back to top
Authorship
Upon submission, the complete author list must be supplied, equally contributing authorships have to be acknowledged and the corresponding author’s contact information provided. The corresponding author accounts for the correct listing of all legitimate contributors as authors and guarantees that all authors have explicitly agreed to the manuscript’s content and its submission to Microbial Cell. Should we become aware of an authorship dispute, we will request a written approval from each author.
Microbial Cell follows the recommendations by the International Committee of Medical Journal Editors (ICMJE) and requires each author to be listed as such who has (i) substantially contributed to the work’s conception or design; or data acquisition, analysis, and/or interpretation AND (ii) drafted or revised the work critically for important intellectual content AND (iii) given her/his final approval of the version to be published AND (iv) agreed to be accountable for all aspects of the work. According to these four criteria, each person qualifying for authorship has to be listed as author and each person listed as author has to qualify as such. Contributors that do not meet authorship conditions should be listed in the acknowledgments section (see also Preparation of Specific Sections). >> Back to top
Conflict of Interest
The authors should indicate any conflicts of interest and sources of financial support. A conflict of interest exists when an author (or the authors‘ institution), reviewer, or editor has financial or personal relationships that inappropriately influence (bias) his or her actions. The potential for conflict of interest can exist whether or not an individual believes that the relationship affects his or her scientific judgment. Financial relationships (such as employment, consultancies, stock ownership, honoraria, paid expert testimony) as well as personal relationships and academic competition must be declared. The authors declare conflicts of interests and sources of financial support in the acknowledgment section (see Preparation of Specific Sections). >> Back to top
Informed consent
Patients have a right to privacy that should not be infringed without informed consent. Identifying information should not be published unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. A patient who is identifiable must be shown the manuscript to be published. Identifying details should be omitted if they are not essential. If identifying details are altered, editors should be informed. When informed consent has been obtained it should be indicated in the published article as: Informed consent has been obtained. >> Back to top
Materials and Data Distribution
Publication with Microbial Cell entails the authors’ agreement to make any material available and provide detailed protocols used in their article upon reasonable request by qualified researchers for the purpose of academic, non-commercial use. If the availability of any article-related information is restricted, this must be communicated in the cover letter and disclosed in the Materials and Methods section of the manuscript upon submission.
If an article describes novel nucleic acid and protein sequences, presents macromolecular, X-ray-determined crystallography (including structure factors), or microarray data, this information must be deposited in the appropriate public repository before publication; the corresponding accession numbers, entry names or digital object identifiers (DOIs) must be published with the paper in the Materials and Methods section. For microarray analyses, see also, Guidelines for specific study designs. Note that violation of Microbial Cell’s “Materials and Data Distribution” policy may lead to retraction of the article. >> Back to top
Guidelines for specific study designs
Authors should take account of any reporting guidelines that are connected to their study design, including the provision of all required supplemental information (checklists, protocols, flowcharts, etc.) upon submission of their manuscript.
Clinical Trials
We follow the WHO definition of a clinical trial. “A clinical trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes. Interventions include but are not restricted to drugs, cells and other biological products, surgical procedures, radiologic procedures, devices, behavioural treatments, process-of-care changes, preventive care, etc”
Microbial Cell supports the International Committee of Medical Journal Editors’ (ICMJE) position on trial registration. All trials initiated after July 1st 2005 must be registered prospectively in a publicly accessible registry (i.e., before patient recruitment has begun), or they will not be considered for publication. For trials initiated before July 1st 2005, all trials must be registered before submission to Microbial Cell. Please, refer to the ICMJE recommendations on trial registration for further details and consult the WHO list of approved registries.
Authors of randomized controlled trials must conform with the CONSORT reporting guidelines that are appropriate to their trial design. These guidelines can be consulted at the CONSORT statement Web site. Before their manuscript can enter peer review authors must (i) mention in their paper trial registry, trial registration number, and the institutional review board (IRB) and (ii) provide a copy of the trial protocol and a completed CONSORT checklist as supporting files. The CONSORT flow diagram must be included as Figure 1 in the manuscript. Any deviation from the trial protocol must be explained in the paper. Authors must explicitly discuss informed consent in their paper, and Microbial Cell reserves the right to ask for a copy of the patient consent form. Information on statistical methods or participants beyond what is indicated in the CONSORT statement should be reported in the Materials and Methods section.
Systematic Reviews and Meta-Analyses of Randomized Controlled Trials
Reports of meta-analyses of randomized controlled trials (RCTs) should use the QUOROM statement as a guide and include the corresponding checklist and flow diagram.
Diagnostic Studies
Reports of studies of diagnostic accuracy should conform to the STARD requirements.
Epidemiological Studies
Authors reporting epidemiological studies should consult the STROBE initiative.
Microarray Experiments
Reports of microarray experiments should adhere to the MIAME guidelines and the data from the experiments must be deposited in a publicly accessible database. >> Back to top
Human and Animal Research
All research involving humans and animals must have been approved by the authors’ institutional review board or equivalent committee and that board must be named by the authors.
Human participants
Informed consent must have been obtained and all clinical investigation must have been conducted according to the principles expressed in the Declaration of Helsinki. Authors should provide a statement from the ethics committee or institutional review board indicating their approval of the research. Microbial Cell also encourages authors to submit a sample of a patient consent form and may require submission of completed forms on particular occasions.
For studies involving humans categorized by race/ethnicity, age, disease/disabilities, religion, sex/gender, sexual orientation, or other socially constructed groupings, authors should – as much as possible – (i) explicitly describe how human populations were categorized, (ii) define categories in as much detail as the study protocol allows, (iii) provide a justification why categories and definitions were chosen (e.g. if specific categorization rules were demanded by the funding agency), (iv) explain whether (and if so, how) they controlled for confounding variables such as socioeconomic status, nutrition, environmental exposures, etc. and (v) avoid outmoded terms and potentially stigmatizing labels and use more current, acceptable terminology.
Animal studies
All work using animals must have been conducted according to applicable national and international guidelines. Prior, approval must have been obtained for all protocols from the relevant authors’ institutional or other appropriate ethics committee, and the institution name and permit numbers must be provided at submission in a statement of ethical approval (provide this statement at the beginning of the Materials and Methods section). For research involving non-human primates, all studies must be performed in accordance with the recommendations of the Weatherall report (2006) and provide details regarding animal welfare and specific steps taken to alleviate suffering. >> Back to top
Standards
The use of established standards allows the reliable integration and linking of scientific information. Thus, Microbial Cell enforces the employment of such standards as applied in the scientific and medical fields in general and in the field of unicellular research in particular.
These standards include, for instance, the use of established nomenclature, which generally includes: (i) the use of SI units, (ii) the italicization of species names (e.g., Saccharomyces cerevisiae), writing out the full genus and species at the first mention of an organism in a paper (if it appears in the title, there as well), after which the first letter of the genus name, followed by the full species name may be used, (iii) the application of recommended gene names according to the appropriate genetic nomenclature database of that organism (if synonyms exist, you might indicate them upon the first mention of the gene in the text) and (iv) the provision of the recommended International Nonproprietary Name (rINN) of drugs.
Among these standards is also the indication of accession numbers to public resources that hold datasets, images, or information mentioned in the article. These should be provided in parentheses after the corresponding piece of information on first use. Suggested databases include, but are not limited to: ArrayExpress, BioModels Database, Database of Interacting Proteins, DNA Data Bank of Japan [DDBJ], DRYAD, EMBL Nucleotide Sequence Database, GenBank, Gene Expression Omnibus [GEO], Protein Data Bank, UniProtKB/Swiss-Prot, and ClinicalTrials.gov. In addition, please provide accession numbers or identifiers for all entities such as genes, proteins, mutants, diseases, etc., for which there is an entry in a public database, such as Ensembl, Entrez Gene, FlyBase, InterPro, Mouse Genome Database (MGD), Online Mendelian Inheritance in Man (OMIM), or PubChem. >> Back to top
Preparation and submission of manuscripts
A detailed description on how to prepare and submit a manuscript is provided in our guidelines for preparation of a manuscript and our the guidelines for submission of a manuscript, respectively. Note that Microbial Cell follows the recommendations by the International Committee of Medical Journal Editors (ICMJE) regarding best practice in manuscript preparation and submission. >> Back to top
Press Coverage of Accepted Papers
As of the day of acceptance in Microbial Cell, authors are free to discuss their work with the general press, their institution’s press office (e.g. to issue a press release) and/or other scientific journals for general coverage and/or report in review or commentary material. Note, that journalists that would like to be added to Microbial Cell‘s press list to be updated on recently accepted articles, should contact us via e-mail. >> Back to top
Scientific misconduct and retraction
According to the International Committee of Medical Journal Editors (ICMJE) scientific misconduct includes but is not necessarily limited to data fabrication; data falsification including deceptive manipulation of images; and plagiarism when scientific misconduct is alleged, or concerns are otherwise raised about the conduct or integrity of work described in submitted or published papers, Microbial Cell initiates appropriate procedures as detailed by the Committee on Publication Ethics (COPE). As the case requires, the Editors may choose to publish an expression of concern pending the outcomes of those procedures. If the procedures involve an investigation at the authors’ institution, the Editors will seek to discover the outcome of that investigation, notify readers of the outcome if appropriate, and if the investigation proves scientific misconduct, publish a retraction of the article. If misconduct remains unproven, the Editors may still publish an expression of concern (together with the exchange of letters to the editor) to highlight matters of debate to readers.
Expressions of concern and retractions will be prominently labeled, appear as a properly numbered article to ensure proper indexing, and include in their heading the title of the original article. In addition, the retraction and original article will be linked in both directions and the retracted article clearly labeled as retracted in all its forms (HTML, PDF). Ideally, the authors of the retraction should be the same as those of the article, but if they are unwilling or unable, the Editors may under certain circumstances accept retractions by other responsible persons, or the Editors-in-Chief may be the sole authors of the retraction or expression of concern. The text of the retraction will explain why the article is being retracted and include a complete citation reference to that article. Retracted articles will remain in the public domain and be clearly labeled as retracted.
In the case of a fraudulent paper, the publishers of Microbial Cell (Shared Science Publishers) will ask the author’s institution to assure them of the validity of other work published in Microbial Cell or other journals by Shared Science Publishers, or they may retract it. If this is not done, an announcement expressing concern that the validity of previously published work is uncertain may be published. >> Back to top
Note: These editorial policies are partly based on those by the open access journals Aging and PLOS Biology as well as on the recommendations by the International Committee of Medical Journal Editors (ICMJE).