Back to article: RidA proteins contribute to fitness of S. enterica and E. coli by reducing 2AA stress and moderating flux to isoleucine biosynthesis
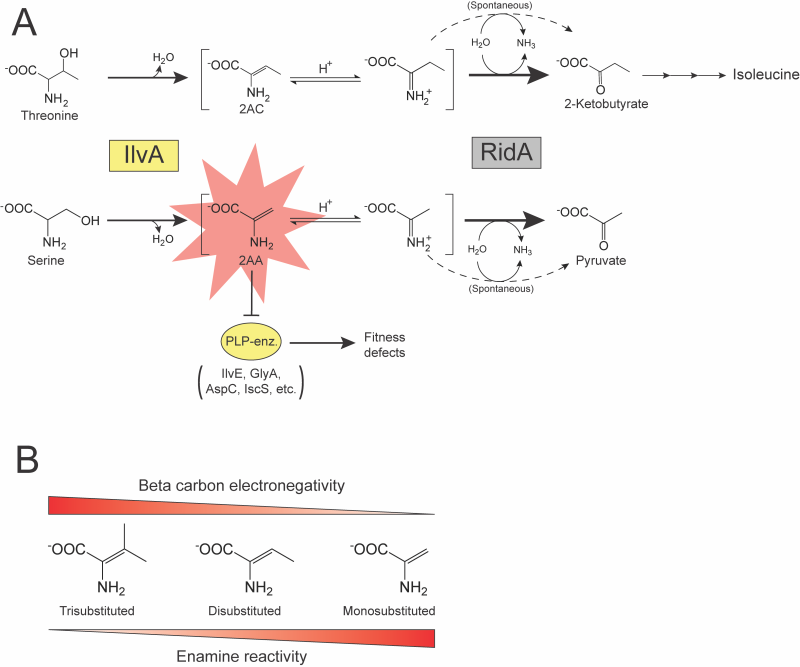
FIGURE 1: RidA paradigm and the effect of pi bond substitution on enamine reactivity. (A) RidA paradigm. IlvA (Serine/threonine dehydratase; EC:4.3.1.19) (yellow box) uses either threonine or serine as a substrate to generate 2AC or 2AA (red star), respectively. Enamine intermediates are spontaneously deaminated by water (dashed arrows) or by RidA (2-iminobutanoate/2-iminopropionate deaminase; EC:3.5.99.10) (grey box, bold arrows). 2AC is a disubstituted and benign enamine and has not been shown to damage cellular components in the absence of RidA. 2AA is a less substituted, more reactive enamine that can damage PLP-dependent enzymes (yellow oval), causing fitness defects if it persists in the cell. (B) Substitution of the pi bond between the α– and β-carbons increases stability of amino acid-derived enamines via increased electronegativity of the β-carbon.