Reviews:
Microbial Cell, Vol. 6, No. 8, pp. 324 - 334; doi: 10.15698/mic2019.08.685
The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging
1 Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America.
2 Center for Gastrointestinal Biology and Disease, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America.
3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America.
# Both first authors contributed equally to this chapter.
Keywords: microbiota, aging, chronic inflammation, cancer, immune development, immune maturation, microbiome, immunosenescence.
Abbreviations:
CRC – colorectal cancer,
GF – germ-free,
IBD – inflamma-tory bowel disease,
IL – interleukin,
iNKT – invariant natural killer T,
NK – natural killer,
NKT – natural killer T,
ROS – reactive oxygen species,
Th – T helper,
Treg – regulato-ry T cell.
Received originally: 13/08/2018 Received in revised form: 23/10/2018
Accepted: 13/11/2018
Published: 13/05/2019
Correspondence:
Janelle C. Arthur, University of North Carolina at Chapel Hill 125 Mason Farm Rd CB#7290 Chapel Hill, North Carolina, 27599, USA, 919-966-2206; janelle_arthur@med.unc.edu
Conflict of interest statement: We report no conflict of interest.
Please cite this article as: Taylor N. Tibbs, Lacey R. Lopez, and Janelle C. Arthur (2019). The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microbial Cell 6(8): 324-334. doi: 10.15698/mic2019.08.685
Abstract
From birth, the microbiota plays an essential role in human development by educating host immune responses. Proper maturation of the immune system perturbs chronic inflammation and the pathogenesis of disease by preventing inappropriate immune responses. While many have detailed the roles of specific microbial groups in immune development and human disease, it remains to be elucidated how the microbiota influences the immune system during aging. Furthermore, it is not yet understood how age-related changes to the microbiota and immune system influence the development of age-related diseases. In this review, we outline the role of the microbiota in immune system development as well as functional changes that occur to immune cell populations during immunosenescence. In addition, we highlight how commensal microbes influence the pathogenesis of cancer, a prominent disease of aging. The information provided herein suggests that age-related changes to the microbiota and immune system should be considered in disease treatment and prevention strategies.
INTRODUCTION
Our microbiota is an integral part of our mammalian selves. Indeed, all multi-cellular eukaryotic hosts across the tree of life have an essential and characteristic microbiota that influences host development and resistance to disease. Complex organisms, such as vertebrates, host numerous microbial communities whose composition and function are relevant to their habitat at different body sites, such as the intestines (“gut”), skin, and oral cavity. The human gut microbiota is perhaps the best studied, most abundant, and arguably, the most influential microbiota that impacts host phenotypes [1]. In recent decades, the development of several scientific tools has exponentially increased our understanding of the microbiota and interactions with its human host. These include model organisms, most notably laboratory mice, that are born and raised germ-free (GF) and then colonized with known individual strains or groups of microbes – “gnotobiotic”. Through the use of GF and gnotobiotic mice, we have been able to demonstrate causality of specific microbes and microbial groups with distinct processes on immune development and non-infectious diseases like chronic inflammation and cancer, among others [1][2][3]. To validate the physiologic relevance of observations made in model organisms with human disease, we can now survey the human microbiota at an unprecedented depth using culture-independent molecular methods (i.e. targeted 16S “microbiome” sequencing, metagenomics, metatranscriptomics, and metabolomics) coupled with sophisticated bioinformatics pipelines. An important finding from population studies of the microbiome has revealed that the compositional fluctuations in an individual's microbiome over time are less substantial than inter-individual differences at a particular stage in development. However, the developmental changes that occur during early life and over an individual's lifespan certainly shape the composition and function of the microbiota [4]. On the other hand, the composition and functional capabilities of the microbiota shape host development [1]. In this review we discuss the current state of knowledge regarding the influence of our mammalian microbiota on the immune system, chronic inflammation, and a prominent disease of the aging – cancer.
THE IMMUNE SYSTEM AND MICROBIOTA IN AGING
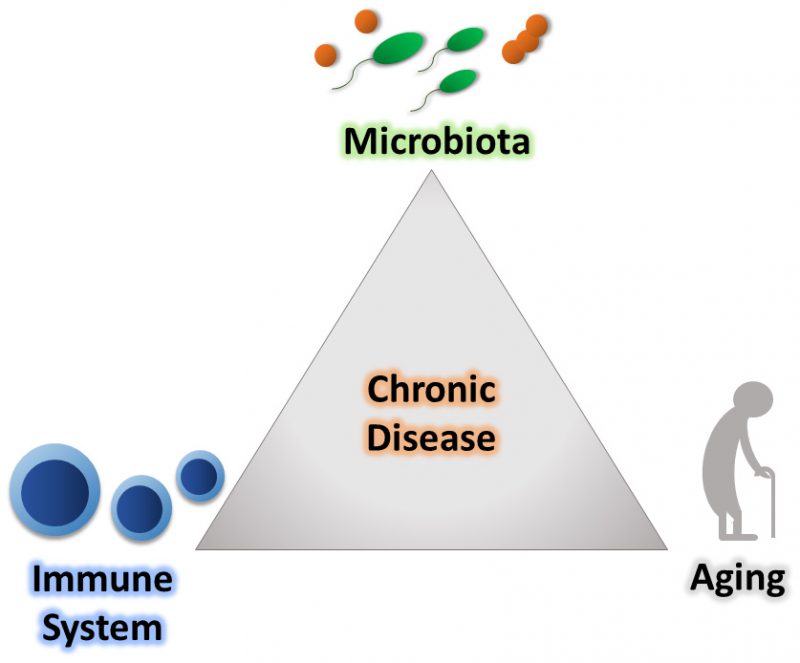
The immune system is our major host defense system that is educated early in life to distinguish harmful stimuli, including microorganisms. It is broadly subdivided into two branches, the innate and adaptive immune systems. The innate immune response is the first line of defense towards invading pathogens. For example, this branch of the immune system is involved when conserved pathogen-specific molecules trigger host cell pattern recognition receptors, which promotes an immediate and broad-spectrum protective response to pathogens. Conversely, the vertebrate adaptive immune response relies upon binding of unique antigens to specialized receptors, which ultimately leads to activation of T and B lymphocytes and the creation of pathogen-specific immunological memory. Through precise coordination, these systems provide host defense against foreign invaders. However, to efficiently mediate its response, the immune system must accurately distinguish between resident host-associated organisms and those that are potentially deleterious [5]. Over the course of our lifetime, our immune system encounters a diverse range of stimuli in a variety of contexts, which challenges the ability of the immune system to differentiate between self and non-self [6]. Alterations in this response can result in the development of a variety of diseases that include chronic inflammatory diseases, like inflammatory bowel disease (IBD) and cancer [7][8]. As these immune responses are shaped over a lifetime, it indicates that age impacts the recognition of stimuli, which could transfer towards inappropriately reacting to residential host microbes. Inappropriate reactions to resident commensal bacteria may underlie a variety of pathogenic processes associated with aging (Figure 1).
–
–
Microbial influences on immune development
Establishment of the microbiota begins from birth and continues until post-weaning [9]. During establishment, the microbiota is highly diverse and prone to fluctuations based on environmental and dietary changes [10]. After 2-3 years of age, this complex community stabilizes with the majority of bacterial community members remaining unchanged throughout an individual's lifespan [11]. After stabilization, the core human microbiota mainly comprises the following phyla: Bacteroidetes and Firmicutes, with a smaller abundance of Actinobacteria, Proteobacteria, and Verrucomicrobia [12]. The aging process strongly impacts the composition of the microbiota as individuals with increased frailty, an assessment of biological age based on current health status and life expectancy, lose bacterial diversity and form a Bacteroidetes dominant population [13]. This loss of microbial diversity may potentiate aging and disease. Indeed, an aging model in turquoise killifish reported that older fish had a marked decrease in gut microbial diversity which favored more pathogenic genera [14]. Moreover, transferring the gut microbiota of these young turquoise killifish into middle-aged fish helped retain species diversity and significantly increased overall lifespan [14]. While not directly correlated to chronological aging, this loss of bacterial diversity most often begins in humans between 75-80 years of age and is a form of dysbiosis that potentiates disease development [15]. These results are a generality derived from a diverse population, and only consider numerical age (lifespan) rather than the individual's aging-related health status (healthspan) [16]. Despite this correlation, it is important to remember that the microbiota play a largely protective role in disease development by initiating and educating the host immune system [17].
–
Early observations in GF mice demonstrated that the host microbiota is essential for the maturation of the immune system [17][18]. In the absence of a microbiota, GF mice have several immunological defects, including reduced lymphoid cell numbers and function [19]. For example, GF mice have fewer T helper type 1 (Th1) cells compared to their conventionalized counterparts [20]. Th1 cells promote cell-mediated immune responses and phagocyte-dependent inflammation to target intracellular pathogens [21]. Th1 responses in GF mice can be restored through host colonization with a variety of microbes, including the well-studied pathogen Listeria monocytogenes, which promotes Th1 development through macrophage production of the T cell-stimulating factor, interleukin 12 (IL-12) [22]. Intracellular bacteria like L. monocytogenes specifically induce Th1 responses in the gut [23]. Additionally, GF mice have a reduced number of T helper type 17 (Th17) cells. Th17 cells are generally pro-inflammatory, however, they drive production of IL-17 and mediate defense against extracellular pathogens and autoimmune disease [20][21]. Adherent bacteria, such as Clostridia-related segmented filamentous bacteria (SFB), induce the development of Th17 cells in the small intestine by driving the release of serum amyloid A from intestinal epithelial cells (IECs). The release of serum amyloid A results in the production of innate lymphoid cell group 3 (ILC3) cytokines which upregulate the Th17 response [24]. Fine-tuning of Th1 and Th17 responses are essential for immune tolerance towards the host microbiota, as seen in the case of IBD where aberrant populations of Th1 and Th17 cells lead to enhanced pathology [7]. Underdevelopment of these responses may underlie the progression of other diseases associated with chronic inflammation, such as cancer [25][26].
–
The absence of a microbiota impacts most, if not all, aspects of the immune system [1]. However, we are just beginning to understand precisely which microbes induce specific effects, and where the window of opportunity lies for correcting many of these immune deficiencies. One study examining colonic invariant natural killer T (iNKT) cell populations revealed that this opportunity for modulation likely occurs during infancy, prior to weaning [27]. At birth, GF mice have an enriched population of colonic lamina propria iNKT cells compared to specific-pathogen-free (SPF) mice [28]. iNKT cells are pro-inflammatory and mediate tolerance to commensal microbes [29]. Colonization of adult (>5 weeks of age) GF mice with a complex microbiota does not influence the number or activity of iNKT cells [27]. However, if the colonization occurs when GF mice are neonates, the number of iNKT cells is reduced and their later activation is well-controlled [27]. This early education of the colonic iNKT cell population is important for limiting morbidity associated with IBD [27]. This supports the idea that exposure to specific microbes and microbial products is needed within a certain developmental time for the host to appropriately educate target immune populations and prevent disease.
–
The immune system and aging
The presence of a microbiota in early life is essential for immune system maturation. However, education of the immune response is a lifelong process. Alterations to the innate and adaptive immune systems which occur with increased frailty are linked to a complex biological process known as immunosenescence [30]. Specific changes associated with immunosenescence can best be understood through functional differences within the unique cell types of the innate and adaptive immune systems. For cells of the innate immune system, there are reported functional differences for every major cell type [31]. However, the most distinct differences are within neutrophil and macrophage populations. Neutrophils isolated from the blood of individuals (aged 62-83 years old) displayed reduced phagocytic capabilities and decreased production of reactive oxygen species (ROS) when infected with Staphylococcus aureus, which correlated with impaired bactericidal activity [32]. Neutrophils are the first line of defense towards invading pathogens; therefore, immunosenescence-related changes to this cell type suggest an age-related decline in pathogen-induced responses and tolerance towards resident microbes [5]. Similarly, primary macrophages isolated from aged mice (18-24 months old) exhibit impaired phagocytosis and reduced ROS production in response to infection, when compared to macrophages isolated from young mice (2-3 months old) [33][34]. Additionally, macrophages from aged mice displayed modifications in antigen presentation and reduced production of pro-inflammatory cytokines [35][36]. Alterations in macrophage antigen presentation and cytokine release may lead to defective immune signaling between the innate and adaptive immune systems, resulting in a weakened immune response [5]. Overall, age-related changes to the innate immune system strongly reduce the host's initial response to pathogens and how the innate system informs adaptive responses. Strategic communication and coordination between these systems is required for proper immune functioning; therefore, maladaptive innate immune responses can misinform the subsequent adaptive immune responses and contribute to the development of disease.
–
While changes in the innate immune system have been noted with aging, long-term effects of the microbiota on adaptive responses are more pronounced [31][37]. The adaptive immune system is used for long-term protection from environmental insult and invading pathogens. Therefore, long-term education of this subsystem could have additive effects on immunosenescence. B cells are a major cell type of the adaptive immune system. The population of antigen-experienced B cells is divided into plasma cells and memory B cells. Plasma cells produce pathogen specific antibodies, while memory B cells provide long-term recognition of antigens via their ability to quickly reactivate upon subsequent antigen encounter [38]. Peripheral blood isolated from elderly individuals (aged 86-94 years old) showed a reduction in B cell population diversity that was attributed to a decrease in memory B cells [39]. This decline in B cell diversity was linked to increased frailty and could be used as a predictor for general health status [39]. A reduction in memory B cell numbers may cause an inappropriate immune response towards the microbiota, as B cells are important for establishing the distinction between pathogenic and commensal bacteria [38]. The reduction in memory B cell numbers that accompanies age may facilitate inappropriate immune responses towards the microbiota, promoting microbial dysbiosis and enhancing disease risk.
–
T cells are the second major cell type of the adaptive immune system and are classified as either conventional or unconventional T lymphocytes [40]. This classification is based on unique T cell surface markers, functional ability, and body site localization [40]. In general, T cells become activated upon binding of an antigen which is displayed on the surface of antigen presenting cells (APC) [41]. Once activated, conventional T cells can perform a wide range of functions from promoting long-term immunity to killing infected cells [41]. During immunosenescence, one of the most prominent changes to occur within conventional T cell populations is the reduction of CD28+ T cells [42]. CD28 is a co-stimulatory protein expressed on naïve T cells and is important for T cell activation, regulation, and survival. Therefore, a reduction in CD28+ T cells may lessen T cell activation causing increased susceptibility to pathogens [43]. Additionally, the loss of CD28 may decrease tolerance to self-antigens and the microbiota as it is also a negative regulator of immune responses [43][44]. To highlight this point, a recent article revealed an age-related reduction in naïve CD8+ T cells, which could compromise host response to pathogens and self-antigens [45]. Conversely, this group found an age-associated increase in memory CD4+ T cells, which corresponds to the cumulative effects of a lifelong antigenic load [45]. Alterations in conventional T cell populations may contribute to chronic inflammation and the onset of age-related disease thorough inappropriate immune responses towards pathogens and the self.
–
While conventional T cells perform a wide range of functions, their role during immunosenescence is complex and not fully detailed. On the other hand, a class of unconventional T cells, known as natural killer T (NKT) cells, are strongly influenced by the microbiota and immunosenescence. In two separate studies, populations of T cells isolated from the peripheral blood of elderly patients showed a reduction in the proportion of NKT cells versus cells isolated from young patients [46][47]. Additionally, NKT cells isolated from the liver of aged mice (aged >20 months old) demonstrated a decline in cytotoxic effector function, and reduced cytokine release versus NKT cells isolated from young mice (aged 2 months old) [48]. This decline in NKT cell number and immunological function may exacerbate disease development by weakening the host's response to pathogens and reducing immunotolerance towards the microbiota.
–
A decrease in the proportion of CD28+ T cells and NKT cells may potentiate the development of autoimmune diseases within elderly populations by reducing tolerance to self-antigens. However, despite an increase in autoantigens within aging individuals, old-age is not a major risk factor for most autoimmune diseases. Studies looking at the proportion of regulatory T cells (Tregs), demonstrate that the repertoire of peripheral Tregs is higher within elderly humans [49]. Since Tregs promote tolerance to self-antigens, the higher proportion of Tregs in aging individuals could be working against perturbations in immunocompetence to prevent autoimmune diseases. More studies are needed to demonstrate age-related changes in Treg functional capacity. Nevertheless, an increased proportion of Tregs is not without some cost as immunosuppression by Tregs may promote chronic infections, reduce vaccine efficiency, and increase rates of cancer among the elderly. However, it remains to be seen what impact the microbiota has on T cell-based immunosenescence in the context of aging.
–
The host microbiota initiates immune system maturation in early life. However, to keep up with a lifelong antigenic load, the immune response must be fine-tuned and properly educated across the lifespan. Despite these observations, it remains unclear how age-related immunological changes impact cellular crosstalk and overall immunocompetence. On top of this, how the microbiota impacts the immune system during immunosenescence remains to be elucidated. It is likely that changes to the immune system result in an inappropriate response towards commensal microbes, as indicated by diseases like IBD [3][50]. Inappropriate reactions to the native microbiota and lessened ability to control invading pathogens may contribute to the development of chronic inflammation and the onset of age-related diseases, such as cancer [51].
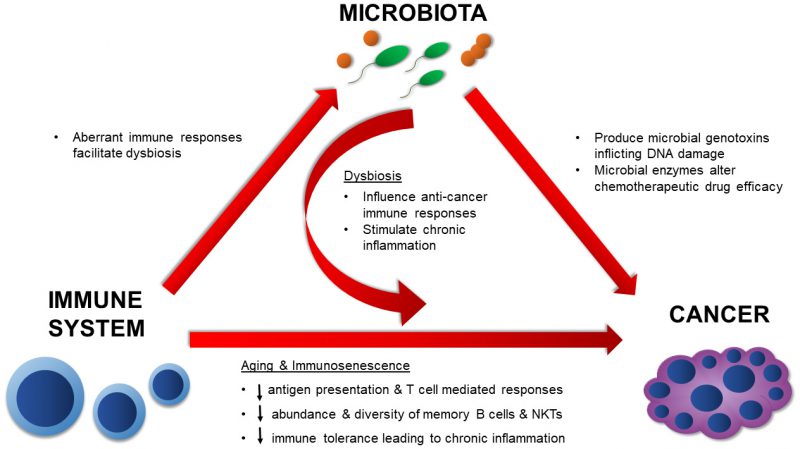
MICROBIOTA AND CANCER – A DISEASE OF AGING
Cancer is considered a disease of old age. As life expectancy increases, the estimated rate of cancer is predicted to increase by 45% from 2011 to 2030 in the United States [52]. It is also estimated that by 2030, individuals 65 years and older will contribute to 70% of all cancers in the U.S. [53]. The risk of developing cancer increases dramatically with age as the duration of time in which an individual is exposed to carcinogens increases [54], the proliferative capacity of aging cells decreases [55], and immunological competence decreases [56]. Cancer typically results from a series of genetic mutations or epigenetic modifications that develop sequentially overtime [57]. The colon, which harbors the largest and most diverse microbiota of all organs, has the highest incidence rate of all reported cancers in the 85+ population [58]. Over the past decade, we have become increasingly aware of the roles that the microbiota play in the development of cancer and modulation of cancer therapies. We have also elucidated several mechanisms underlying the microbial influences on cancer. However, these roles are diverse and seem to influence many aspects of immune and cancer development [59]. Microbes can contribute to the onset and progression of cancer through direct means, such as by producing genotoxins, and indirect means through the modulation of immune responses to tumors and immunotherapy [60][61][62][63][64]. Additionally, several members of the native microbiota can alter chemotherapeutic drugs, resulting in morbid side effects for the host or even rendering them clinically inert [65][66]. It is therefore important to divulge the significance of microbial interactions on age-related diseases, such as cancer, in order to fully understand disease progression and design suitable therapies. Here we will discuss known mechanisms by which the microbiota can influence the onset of cancer, endogenous anti-cancer immune responses, chemotherapeutic activities, and anti-tumor immunotherapy.
–
Endogenous anti-cancer immune responses
The ability to manipulate the microbiota using GF and gnotobiotic mice has demonstrated the importance of the microbiota on immune system development and the gastrointestinal environment. A notable example is microbial modulation of bile acid composition, which can influence immune responses and also affect the development of malignancies. Host-derived primary bile acids are converted into secondary bile acids by the gut microbiota, primarily by members of the genus Clostridia, and circulated systemically throughout the body via hepatic circulation [67]. Previous work illustrated that secondary bile acids can increase the risk of obesity-associated hepatocellular carcinoma in susceptible mice [68]. Recent data suggests that antibiotic elimination of the gut microbiota in mice decreases both primary and metastatic tumors within the liver by facilitating the buildup of primary bile acids, which trigger liver-specific NKT cell recruitment to target cancer cells [69]. However, the influences of Clostridia spp. on the development of cancer are likely more complex. Treatment of colorectal cancer (CRC)-prone mice with the probiotic cocktail VSL#3, a mixture of lactic acid-producing bacteria with anti-inflammatory properties, decreased the population of Clostridia spp. in the gut and subsequently enhanced tumorigenesis, suggesting that some Clostridia spp. may also be protective against the onset of malignancies [70]. These data highlight how further understanding the conditions by which particular species promote or perturb the development of cancer must be addressed when assessing cancer risk, prevention, and treatment. The profound effects the bacteria elicit on cytotoxic immune cells and tumor development provide key insights on how the native microbiota influence host anti-cancer responses.
–
Fusobacterium nucleatum, a Gram-negative oral commensal overrepresented in CRC, can promote tumorigenesis via direct effects on the epithelium and through the modulation of endogenous immune responses [62][71]. A known target is the natural killer (NK) cell, which kills compromised host cells, such as infected or cancerous cells. F. nucleatum inhibits the cytotoxicity of NK cells via the Fusobacterium protein Fap2, which binds the NK cell inhibitor receptor TIGIT (T cell immunoglobulin and ITIM or immunoreceptor tyrosine-based inhibition motif domain) [72]. In addition to targeting the immune system, F. nucleatum exerts procarcinogenic activities directly on epithelial cells through β-catenin signaling, altering proliferation and cell fate [73]. F. nucleatum can also alter the efficacy of chemotherapeutic drugs by inhibiting host cell apoptotic pathways [74]. F. nucleatum is a prime example of one species of the microbiota that exhibits a variety of different effects on the host to mediate tumorigenesis and hinder cancer therapy. In the next few sections, we will discuss a variety of known bacterial mechanisms that act upon cancer development and treatment.
–
Cancer immunotherapy, the microbiota, and aging
Several independent groups have recently demonstrated that some members of the microbiota play critical roles in determining patient responsiveness to cancer immunotherapy. The exact mechanisms by which individual species of bacteria exhibit these effects are not fully understood. However, current data suggest that bacterial modulation of the immune system may be one critical mode of altering host response to cancer therapy. Recent data regarding anti-PD1 therapy supports this notion. Anti-PD1 treatment is a type of immune checkpoint inhibitor that enhances anti-tumor immune responses by maintaining T cell activation via blocking the immune inhibitory receptors programmed death ligand-1 and 2 (PDL-1 and PDL-2) [75]. Anti-PD1 therapy is often prescribed to patients with lung cancer and advanced melanoma. However, the efficacy of anti-PD1 immunotherapy ranges from only 19 to 43% for both cancer types [76][77]. Several members of the microbiota are enriched in PD-1 responders, including Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium [78]. Faecalibacterium, an abundant Gram-positive genus of commensals in the human gut, was also enriched in PD1-responders [61]. Tumor-bearing mice that were given fecal microbiota transplants (FMTs) from PD1-responders exhibited decreased tumor burden and tumor size when receiving anti-PD1 therapy. Faecalibacterium promoted cytotoxic (CD8+) T cell recruitment to tumors, which may be an important mechanism underlying the ability of this bacterial group to enhance anti-PD1 responses and reduce tumor burden [61]. Similarly, FMTs from PD-1 responders enhanced PD-1 treatment in recipient mice, which was further augmented with oral supplementation of the commensal Akkermansia muciniphila [79]. Antibiotic treatment reduced the efficacy of PD-1 immunotherapy in mice, consistent with clinical reports of reduced PD-1 efficacy in patients simultaneously taking antibiotics [79]. These studies demonstrate that multiple species of bacteria have the capability of altering immunotherapeutic responses in patients. Moving forward, it will be critical to consider the contributions of these microbial communities when developing anti-tumor immunotherapies.
–
There is a paucity of data evaluating the combined influences of the microbiota and age in immunotherapeutic outcomes. Some studies have investigated the effects of age on immunotherapy; however, the majority of studies heavily rely on metadata and have found few differences in immunotherapeutic efficacy in relation to age [80]. One metadata study reported improved overall survival from anti-CTL4 treatment, but not anti-PD1 treatment in individuals > 75 years of age [81]. Another study in mice showed that CD40/IL-2 treatment for metastatic renal cell carcinoma increases mortality in aged mice compared to young mice, due to multi-organ failure/systematic toxicity [82]. This study illustrates that immunotherapies, which are commonly developed in young mouse models (2-4 months old), may not take into account the immune changes that occur in aging populations; therefore, the altered immune environment associated with aging should be considered when developing suitable immunotherapeutic strategies. Furthermore, side effects of immunotherapies may be exacerbated in the elderly due to other age-related deficiencies, such as increased risk of dehydration from reduced kidney function. The contribution of the microbiota on immunotherapeutic outcomes specifically in aged individuals is unknown. Clinicians should be thoughtful in prescribing immunotherapies to aged individuals and consider immunocompetency changes and individual microbial diversity that may exacerbate side effects or affect the efficacy of immunotherapeutic drugs.
–
Chemotherapy and the microbiota
Multiple members of the microbiota can differentially influence cancer chemotherapy, with some enhancing and some inhibiting the clinical effects of chemotherapeutic drugs. An important early observation was that genotoxic platinum chemotherapies, including oxaliplatin and cisplatin, were ineffective in tumor-bearing GF mice, indicating that the presence of a complex microbiota is essential for these chemotherapies [83]. Platinum chemotherapies promote ROS to induce cytotoxicity. The DNA damage incited by cisplatin is augmented via the production of mitochondrial ROS within tumor-associated inflammatory cells and cancer cells themselves [83][84]. It may be that in the absence of a native microbiota, inflammatory cells are not effectively primed to produce ROS during development, leading to shortcomings in ROS production later in life that may affect the efficacy of platinum-based chemotherapies. These data illustrate that a properly developed immune system trained by the native microbiota augment anti-tumor responses during chemotherapeutic treatment. Furthermore, these insights highlight the influential capacity of the microbiota on the host and how in their absence, the immune system may have substantial deficits in anti-tumor responses.
–
Conversely, the microbiota can have negative effects on chemotherapeutic efficacy. Deep sequencing for microbes within pancreatic tumor biopsies revealed that 57.5% of pancreatic tumor tissues tested (65 of 113 samples) were positive for bacterial reads, with Gammaproteobacteria being the most abundant (51.7% of reads) [66]. Interestingly, 98.4% of Gammaproteobacteria contain genes that encode a specific isoform of the enzyme cytidine deaminase (CDDL), which has the ability to break down gemcitabine and confer chemotherapeutic resistance in tumor tissues [66]. Accordingly, bacterial migration from the gastrointestinal tract into the pancreatic ducts and tumor tissue may be a significant source of drug failure in clinical pancreatic cancer cases. Gut bacteria are also responsible for re-activating chemotherapeutic drugs in the distal intestine. Irinotecan, a chemotherapeutic drug used to treat CRC, is inactivated by the liver, but reactivated into the active drug by Clostridia spp. through bacterial β-glucuronidases in the gut [85]. This re-activation in the distal intestine contributes to the typical morbid gastrointestinal side effects of irinotecan therapy, including mucositis and diarrhea [65][85]. This evidence illustrates the profound impact the native microbiota can have on the response to cancer therapies. Therefore, future treatment plans should account for the influence of these patient-specific microbial factors to ensure successful chemotherapeutic outcomes.
–
Direct effects of the microbiota on tumorigenesis
Specific members of the microbiota have the capacity to directly contribute to tumorigenesis [2][59]. Commensal Enterobacteriaceae, including several strains of Escherichia coli, are capable of inducing DNA damage in mammalian cells by producing a genotoxin termed colibactin [60][86]. The bacterial polyketide synthase (pks) pathogenicity island encoding colibactin is upregulated in CRC mouse models and the presence of these gene products promotes tumorigenesis by inducing double-stranded DNA breaks [87][88][89]. Colibactin can also induce premature cellular senescence in cells that initially survive the DNA damage [90][91]. Furthermore, the pks pathogenicity island is overrepresented in the microbiota of CRC and IBD patients, who represent a population at high risk of developing CRC [60][92][93]. In another population at high risk for CRC, familial adenomatous polyposis patients, pks+ bacteria are found in combination with other pro-carcinogenic microbes in colonic biofilms [94]. This suggests that bacterial genotoxins contribute substantially to the risk and development of chronic inflammatory diseases and human cancer.
–
Gut bacteria also have the capacity to induce a pro-tumorigenic environment through chronic inflammation. Enterotoxigenic Bacteroides fragilis, a member of the most abundantly represented genus in the gut, produces its own flavor of toxin called B. fragilis-derived toxin (BFT) [95]. BFT is a zinc-dependent metalloprotease that can induce colitis and promote tumorigenesis through the generation of ROS and subsequent initiation of DNA damage in epithelial cells [96]. Enterotoxigenic B. fragilis robustly activates Th17 immune responses, which involves the inflammatory cytokine IL-17, and may lower host anti-tumor immune responses, encouraging unhindered tumor growth [97][98]. Enterotoxigenic B. fragilis is overrepresented in patients with CRC when compared to healthy individuals [64] and exacerbates tumorigenesis in susceptible mice [98]. Interestingly, the tumorigenic effects of pks+ E. coli and enterotoxigenic B. fragilis act synergistically in vivo to quicken tumor onset and increase mortality in susceptible mice beyond the capability that either species has individually [94]. Given that the native microbial community is quite complex, the cumulative effects of microbial products on the host may significantly contribute to the onset and progression of cancer.
–
While widely considered a pathogen, Helicobacter pylori is estimated to be present in the gastrointestinal tract of over half of the human population worldwide and a major risk factor for gastric adenocarcinoma [99][100]. H. pylori was one of the earliest identified microbial suspects of inflammation-mediated cancer development and it is estimated that H. pylori infection increases the attributable risk of gastric cancer by 73% [101]. Chronic H. pylori infection results in inflammation and tissue damage by the bacterial virulence factor CagA (cytotoxin-associated gene A), which initiates the development of the hallmark precursory lesions of gastric cancer, including intestinal metaplasia and dysplasia [102]. It remains unclear why H. pylori infection only progresses to malignancy in a subset of infected individuals; however, it is postulated that host immune responses and the genetics of both host and microbiota contribute to neoplastic development [103].
–
In summary, the mechanisms by which the native microbiota influences cancer development and therapy are numerous and diverse (Figure 2). The evidence presented here illustrates the diverse microbial mechanisms that contribute to tumorigenesis, whether that be by directly targeting the DNA for damage through a toxin or by providing an augmented environment for unrestricted cellular proliferation. Microbial effects on the immune system are undoubtedly involved in these processes. As more data surfaces, it will be imperative to synthesize and apply knowledge on positive and negative microbial contributions towards cancer development and treatment. By doing so, we can more effectively assess cancer risk and ultimately design more potent anti-cancer therapies.
–
CONCLUSION
Our microbiota plays a central role in human health by educating our immune responses to recognize self versus non-self, across the lifespan. Immune system development begins at birth, with the introduction of the microbiota, and only fully matures in the presence of commensal microbial flora. Proper maturation of the immune system is necessary to prevent aberrant immune responses, which can lead to chronic inflammation and the onset of disease. It is well understood that fine-tuning the immune response is a lifelong educational process; therefore, it is important to consider how the microbiota and immune system change throughout aging. While studies in this review focus on changes linked to chronological age, it is necessary to consider how biological age (assessed by health status and life expectancy) shapes the microbiota and immune system. As we highlight here, a reduction in microbial diversity is linked to increased frailty. Additionally, all major cell types of the innate and adaptive immune systems are functionally altered in the context of aging by a process known as immunosenescence. Despite these marked differences, not much is known about the connection between the microbiota and immune-senescence. Traditionally, studies have focused on classic pathogens and the burden of a lifelong antigenic load as they relate to the improvement of vaccine efficiency in the elderly. However, decreased ability to fight off foreign pathogens is not the only concern of aging. In fact, biological age puts elderly at risk for a wide range of age-related diseases, including cancer, cardiovascular disease, and Alzheimer's disease, all of which have been shown to be influenced by the microbiota [2][3][104][105][106][107][108] While in this review we focus upon how the microbiota and immune system influence the pathogenesis of cancer, it is worth considering the other changes that occur physiologically with aging, how this impacts our microbiota, and vice versa. There is no universal microbiota composition known to mediate inflammation or anti-tumor responses. This is because the microbiota and immune system are unique to each individual and cultivated over a lifespan. Therefore, biological age should be considered in pre-clinical models, as age-related factors will likely affect therapeutic efficacy and outcomes. Age-related changes influence both the host and microbiota; therefore, they should be considered during the design of animal and human studies to provide a more holistic understanding of disease treatment and prevention strategies.
REFERENCES
- Round JL, and Mazmanian SK (2009). The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol 9(5): 313–323. doi: 10.1038/nri2515
- Schwabe RF, and Jobin C (2013). The microbiome and cancer. Nat Rev Cancer 13(11): 800–812. doi: 10.1038/nrc3610
- Sartor B, and Wu GD (2017). Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 152(2): 327–339. doi: 10.1053/j.gastro.2016.10.012
- Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, Knight R, and Ley RE (2014). Conducting a microbiome study. Cell 158(2): 250–262. doi: 10.1016/j.cell.2014.06.037
- Chaplin DD (2010). Overview of the Immune Response David. J Allergy Clin Immunol 125(2 Suppl 2): S3-23. doi: 10.1016/j.jaci.2009.12.980
- Ponnappan S, and Ponnappan U (2011). Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid Redox Signal 14(8): 1551–1585. doi: 10.1089/ars.2010.3228
- Gálvez J (2014). Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 1–14. doi: 10.1155/2014/928461
- Narendra BL, Reddy KE, Shantikumar S, and Ramakrishna S (2013). Immune system: A double-edged sword in cancer. Inflamm Res 62(9): 823–834. doi: 10.1007/s00011-013-0645-9
- Bergström A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Mølgaard C, Michaelsen KF, and Licht TR (2014). Establishment of Intestinal Microbiota during Early Life: a Longitudinal, Explorative Study of a Large Cohort of Danish Infants. Appl Environ Microbiol 80(9): 2889–2900. doi: 10.1128/AEM.00342-14
- Penders J, Thijs C, Vink C, Stelma F oekj. F, Snijders B, Kummeling I, van den Brandt PA, and Stobberingh EE (2006). Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 118(2): 511–521. doi: 10.1542/peds.2005-2824
- Rajilić-Stojanović M, Heilig HGHJ, Tims S, Zoetendal EG, and de Vos WM (2013). Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol 15(4): 1146–1159. doi: 10.1111/1462-2920.12023
- Arumugam M et al. (2011). Enterotypes of the human gut microbiome. Nature 473: 174–180. doi: 10.1038/nature09944
- Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, and O'Toole PW (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci 108: 4586–4591. doi: 10.1073/pnas.1000097107
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, and Valenzano DR (2017). Regulation of life span by the gut microbiota in the short-lived african turquoise killifish. Elife 6(e27014): 1–26. doi: 10.7554/eLife.27014
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, and De Vos W (2010). Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians Elena. PLoS One 5(5): e10667. doi: 10.1371/journal.pone.0010667
- Hansen M, and Kennedy BK (2016). Does Longer Lifespan Mean Longer Healthspan? Trends Cell Biol 26(8): 565–568. doi: 10.1016/j.tcb.2016.05.002
- Pickard JM, Zeng MY, Caruso R, and Núñez G (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease Joseph. Immunol Rev 279(1): 70–89. doi: 10.1111/imr.12567
- Clavel T, Gomes-Neto JC, Lagkouvardos I, and Ramer-Tait AE (2017). Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol Rev 279: 8–22. doi: 10.1111/imr.12578
- Fiebiger U, Bereswill S, and Heimesaat MM (2016). Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol 6(4): 253–271. doi: 10.1556/1886.2016.00036
- Wu H-J, and Wu E (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3(1): 4–14. doi: 10.4161/gmic.19320
- Damsker JM, Hansen AM, and Caspi RR (2010). Th1 and Th17 cells: Ann N Y Acad Sci 1183: 211–221. doi: 10.1111/j.1749-6632.2009.05133.x
- Hsieh C, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, and Murphy KM (1993). Development of Th1 CD4+ T Cells Through IL-12 Produced by Listeria-Induced Macrophages. Science 260(5107): 547–549. doi: 10.1126/science.8097338
- Atarashi K et al. (2015). Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163: 367–380. doi: 10.1016/j.cell.2015.08.058
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch S V., Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, and Littman DR (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139(3): 485–498. doi: 10.1016/j.cell.2009.09.033
- Vinay DS et al. (2015). Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 35: S185–S198. doi: 10.1016/j.semcancer.2015.03.004
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, and Paulos CM (2014). Th17 cells in cancer: The ultimate identity crisis. Front Immunol 5(276): 1–13. doi: 10.3389/fimmu.2014.00276
- An D, Oh SF, Olszak T, Neves JF, Avci F, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, and Kasper DL (2014). Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156(0): 123–133. doi: 10.1016/j.cell.2013.11.042
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, and Blumberg RS (2012). Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336(6080): 489–493. doi: 10.1126/science.1219328
- Chandra S, and Kronenberg M (2015). Activation and Function of iNKT and MAIT Cells. Adv Immunol 127:145-201. doi: 10.1016/bs.ai.2015.03.003
- Fülöp T, Dupuis G, Witkowski JM, and Larbi A (2016). The Role of Immunosenescence in the Development of Age-Related Diseases. Rev Investig CLÍNICA 68(2): 84–91. PMID: 27103044
- Castelo-Branco C, and Soveral I (2014). The immune system and aging: a review. Gynecol Endocrinol 30(1): 16–22. doi: 10.3109/09513590.2013.852531
- Wenisch C, Patruta S, Daxböck F, Krause R, and Hörl W (2000). Effect of age on human neutrophil function. J Leukoc Biol 67(1): 40–45. doi: 10.1002/jlb.67.1.40
- Swift ME, Burns AL, Gray KL, and DiPietro LA (2001). Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117(5): 1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x
- Davila DR, Edwards III CK, Arkins S, Simon J, and Kelley KW (1990). Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J 4(11): 2906–2911. doi: 10.1096/fasebj.4.11.2165948
- Herrero C, Marqués L, Lloberas J, and Celada A (2001). IFN-γ–dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest 107(4): 485–493. doi: 10.1172/JCI11696
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, and Sambhara S (2002). Cutting Edge: Impaired Toll-Like Receptor Expression and Function in Aging. J Immunol 169(9): 4697–4701. doi: 10.4049/jimmunol.169.9.4697
- Linton PJ, and Dorshkind K (2004). Age-related changes in lymphocyte development and function. Nat Immunol 5(2): 133–139. doi: 10.1038/ni1033
- Eibel H, Kraus H, Sic H, Kienzler A-K, and Rizzi M (2014). B cell Biology: An Overview. Curr Allergy Asthma Rep 14(434): 1–10. doi: 10.1007/s11882-014-0434-8
- Gibson KL, Wu Y-C, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson B-O, Wikby A, Kipling D, and Dunn-Walters DK (2009). B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 8: 18–25. doi: 10.1111/j.1474-9726.2008.00443.x
- Roberts S, and Girardi M (2008). Clinical and basic immunodermatology: Conventional and Unconventional T Cells. In: Clinical and Basic Immunodermatology. Springer, London. pp. 85-104. doi: 10.1007/978-1-84800-165-7
- Jin B, Sun T, Yu X-H, Yang Y-X, and Yeo AET (2012). The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol 2012: 1–32. doi: 10.1155/2012/836485
- Tu W, and Rao S (2016). Mechanisms underlying T cell immunosenescence: Aging and cytomegalovirus infection. Front Microbiol 7(2111): 1–12. doi: 10.3389/fmicb.2016.02111
- Bour-Jordan H, and Bluestone JA (2002). CD28 function: A balance of costimulatory and regulatory signals. J Clin Immunol 22(1): 1–7. doi: 10.1023/A:1014256417651
- Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, and Abbas AK (1997). Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6(4): 411–417. doi: 10.1016/S1074-7613(00)80284-8
- Zalocusky KA, Kan MJ, Hu Z, Dunn P, Thomson E, Wiser J, Bhattacharya S, and Butte AJ (2018). The 10,000 Immunomes Project: Building a Resource for Human Immunology. Cell Rep 25: 513–522. doi: 10.1016/j.celrep.2018.09.021
- DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Pena J, and Solana R (2002). Vα24+ NKT cells are decreased in elderly humans. Exp Gerontol 37: 213–217. doi: 10.1016/S0531-5565(01)00186-3
- Jing Y, Gravenstein S, Chaganty RN, Chen N, Lyerly KH, Joyce S, and Deng Y (2007). Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol 42(8): 719–732. doi: 10.1016/j.exger.2007.01.009
- Mocchegiani E, Giacconi R, Cipriano C, Gasparini N, Bernardini G, Malavolta M, Menegazzi M, Cavalieri E, Muzzioli M, Ciampa AR, and Suzuki H (2004). The variations during the circadian cycle of liver CD1d-unrestricted NK1.1+TCRγ/δ+ cells lead to successful ageing. Role of metallothionein/IL-6/gp130/PARP-1 interplay in very old mice. Exp Gerontol 39: 775–788. doi: 10.1016/j.exger.2004.01.014
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, and Moss PA (2005). The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin Exp Immunol 140: 540–546. doi: 10.1111/j.1365-2249.2005.02798.x
- Sun M, He C, Cong Y, and Liu Z (2015). Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol 8(5): 969–978. doi: 10.1038/mi.2015.49
- Tilg H, Adolph TE, Gerner RR, and Moschen AR (2018). The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 33(6): 1–11. doi: 10.1016/j.ccell.2018.03.004
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, and Buchholz TA (2009). Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 27(17): 2758–2765. doi: 10.1200/JCO.2008.20.8983
- White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, and Henley SJ (2014). Age and cancer risk: A potentially modifiable relationship. Am J Prev Med 46(3 0 1): S7-15. doi:10.1016/j.amepre.2013.10.029
- Harding C, Pompei F, and Wilson R (2012). Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer 118(5): 1371–1386. doi: 10.1002/cncr.26376
- Pompei F, Polkanov M, and Wilson R (2001). Age distribution of cancer in mice: The incidence turnover at old age. Toxicol Ind Health 17: 7–16. doi: 10.1191/0748233701th091oa
- Bonafè M, Barbi C, Storci G, Salvioli S, Capri M, Olivieri F, Valensin S, Monti D, Gonos ES, De Benedictis G, and Franceschi C (2002). What studies on human longevity tell us about the risk for cancer in the oldest old: Data and hypotheses on the genetics and immunology of centenarians. Exp Gerontol 37: 1263–1271. doi: 10.1016/S0531-5565(02)00137-7
- Loeb LA, Loeb KR, and Anderson JP (2003). Multiple mutations and cancer. Proc Natl Acad Sci 100(3): 776–781. doi: 10.1073/pnas.0334858100
- Thakkar JP, McCarthy BJ, and Villano JL (2014). Age-specific cancer incidence rates increase through the oldest age groups. Am J Med Sci 348(1): 65–70. doi: 10.1097/MAJ.0000000000000281
- Fulbright LE, Ellermann M, and Arthur JC (2017). The microbiome and the hallmarks of cancer. PLoS Pathog 13(9): 1–6. doi: 10.1371/journal.ppat.1006480
- Arthur JC, Perez-chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, and Jobin C (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338(6103): 120–123. doi: 10.1126/science.1224820
- Gopalakrishnan V et al. (2018). Gut microbiome modulates response to anti – PD-1 immunotherapy in melanoma patients. Science 359(6371): 97–103. doi: 10.1126/science.aan4236
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-omar EM, Brenner D, Fuchs CS, Meyerson M, and Garrett WS (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor immune microenvironment. Cell Host Microbe 14(2): 207–215. doi: 10.1016/j.chom.2013.07.007
- Rhee K-J, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad AR, Gan CM, Housseau F, and Sears CL (2009). Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun 77(4): 1708–1718. doi: 10.1128/IAI.00814-08
- Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, and Sears CL (2015). The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis 60(2): 208–215. doi: 10.1093/cid/ciu787
- Al-Dasooqi N, Bowen JM, Gibson RJ, Logan RM, Stringer AM, and Keefe DM (2011). Irinotecan-induced alterations in intestinal cell kinetics and extracellular matrix component expression in the dark agouti rat. Int J Exp Pathol 92: 357–365. doi: 10.1111/j.1365-2613.2011.00771.x
- Geller LT et al. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357(6356): 1156–1160. doi: 10.1126/science.aah5043
- Ridlon JM, Kang D-J, and Hylemon PB (2006). Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47(2): 241–259. doi: 10.1194/jlr.R500013-JLR200
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, and Ohtani N (2013). Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499(7456): 97–101. doi: 10.1038/nature12347
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, Mcculloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, and Greten TF (2018). Gut microbiome – mediated bile acid metabolism regulates liver cancer via NKT cells. Science 876(May): 1–9. doi: 10.1126/science.aan5931
- Arthur JC, Gharaibeh RZ, Uronis JM, Perez-Chanona E, Sha W, Tomkovich S, Mühlbauer M, Fodor AA, and Jobin C (2013). VSL#3 probiotic modifies mucosal microbial composition but does not reduce colitis-associated colorectal cancer. Sci Re 3(2868): 1–9. doi: 10.1038/srep02868
- Castellarin M, Warren RL, Freeman DJ, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, and Holt RA (2012). Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Elife 22: 299–306. doi: 10.7554/eLife.25801
- Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklić K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, and Mandelboim O (2015). Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack Article Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune. Immunity 42(2): 344–355. doi: 10.1016/j.immuni.2015.01.010
- Rubinstein RM, Wang X, Liu W, Hao Y, Cai G, and Han YW (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14(2): 195–206. doi:10.1016/j.chom.2013.07.012
- Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, and Fang J (2017). Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 170(3): 548–563. doi:10.1016/j.cell.2017.07.008
- Shields BD, Mahmoud F, Taylor EM, Byrum SD, Sengupta D, Koss B, Baldini G, Ransom S, Cline K, Mackintosh SG, Edmondson RD, Shalin S, and Tackett AJ (2017). Indicators of responsiveness to immune checkpoint inhibitors. Sci Rep 7(807): 1–12. doi: 10.1038/s41598-017-01000-2
- Larkin J et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373(1): 23–34. doi: 10.1056/NEJMoa1504030
- Sheng Z, Zhu X, Sun Y, and Zhang Y (2017). The efficacy of anti-PD-1/PD-L1 therapy and its comparison with EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget 8(34): 57826–57835. doi: 10.18632/oncotarget.18406
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, and Gajewski TF (2018). The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359(6371): 104–108. doi: 10.1126/science.aao3290
- Routy B et al. (2018). Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359(6371): 91–97. doi: 10.1126/science.aan3706
- Friedman CF, and Wolchok JD (2017). Checkpoint Inhibition and Melanoma: Considerations in Treating the Older Adult. J Geriatr Oncol 8(4): 237–241. doi: 10.1016/j.jgo.2017.04.003
- Nishijima TF, Muss HB, Shachar SS, and Moschos SJ (2016). Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat Rev 45: 30–37. doi: 10.1016/j.ctrv.2016.02.006
- Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N, Wigginton JM, Wilson SE, Blazar BR, Malyguine AM, Sayers TJ, and Wiltrout RH (2003). Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol 170(5): 2727–33. doi: 10.4049/jimmunol.170.5.2727
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai R, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, and Goldszmid RS (2013). Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 342(6161): 967–971. doi: 10.1126/science.1240527
- Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, and Doetsch PW (2013). Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8(11): 1–15. doi: 10.1371/journal.pone.0081162
- Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh ASJ, Al-Dasooqi N, and Keefe DMK (2009). Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90: 489–499. doi: 10.1111/j.1365-2613.2009.00671.x
- Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, and Nougayrède J-P (2010). Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci 107(25): 11537–11542. doi: 10.1073/pnas.1001261107
- Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, and Jobin C (2014). Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun 1–11. doi: 10.1038/ncomms5724
- Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, Mohamadzadeh M, Liu X, Martin P, Wang GP, Oswald E, Fodor AA, and Jobin C (2017). Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res 77(10): 2620–2632. doi:10.1158/0008-5472.CAN-16-3472
- Nougayrede J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz, Elzbieta Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, and Oswald E (2006). Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science 313(5788): 848–852. doi: 10.1126/science.1127059
- Secher T, Samba-Louaka A, Oswald E, and Nougayrède JP (2013). Escherichia coli Producing Colibactin Triggers Premature and Transmissible Senescence in Mammalian Cells. PLoS One 8(10): 1–17. doi: 10.1371/journal.pone.0077157
- Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Déchelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, and Bonnet R (2014). Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63(12): 1932–1942. doi: 10.1136/gutjnl-2013-305257
- Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, Knight P, Codling C, Marchesi JR, Winstanley C, Hall N, Rhodes JM, and Campbell BJ (2014). Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63(5): 761–770. doi: 10.1136/gutjnl-2013-304739
- Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, and Bonnet R (2013). High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS One 8(2): e56964. doi: 10.1371/journal.pone.0056964
- Dejea CM, Fathi P, Craig JM, Boleij A, Geis AL, Wu X, Shields CED, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, and Sears CL (2018). Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359(6375): 592–597. doi: 10.1126/science.aah3648
- Wu S, Dreyfus LA, Tzianabos AO, Hayashi C, and Sears CL (2002). Diversity of the metalloprotease toxin produced by enterotoxigenic Bacteroides fragilis. Infect Immun 70(5): 2463–2471. doi: 10.1128/IAI.70.5.2463-2471.2002
- Goodwin AC, Shields CED, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, and Casero Jr. RA (2011). Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci 108(37): 15354–15359. doi: 10.1073/pnas.1010203108
- Geis AL, Fan H, Wu X, Wu S, Huso DL, Wolfe JL, Sears CL, Pardoll DM, and Housseau F (2015). Regulatory T cell response to enterotoxigenic Bacteroides fragilis colonization triggers IL-17-dependent colon carcinogenesis. Cancer Discov 5(10): 1098–1109. doi: 10.1158/2159-8290.CD-15-0447
- Wu S, Rhee K, Albesiano E, Rabizadeh S, Wu X, Yen H, Huso DL, Brancati FL, Wick E, Mcallister F, Housseau F, Pardoll DM, and Sears CL (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15(9): 1016–1022. doi:10.1038/nm.2015
- Arthur JC, and Jobin C (2011). The struggle within: Microbial influences on colorectal cancer. Inflamm Bowel Dis 17(1): 396–409. doi: 10.1002/ibd.21354
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, and Ng SC (2017). Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153: 420–429. doi: 10.1111/jgs.12611
- Herrera V, and Parsonnet J (2009). Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect 15(11): 971–976. doi: 10.1111/j.1469-0691.2009.03031.x
- Díaz P, Valenzuela Valderrama M, Bravo J, and Quest AFG (2018). Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front Microbiol 9: 1–10. doi: 10.3389/fmicb.2018.00005
- Polk DB, and Peek RM (2010). Helicobacter pylori: Gastric cancer and beyond. Nat Rev Cancer 10(6): 403–414. doi: 10.1038/nrc2857
- Jaul E, and Barron J (2017). Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Heal 5: 335. doi: 10.3389/fpubh.2017.00335
- Jie Z et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8(845): 1–12. doi: 10.1038/s41467-017-00900-1
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison, Bruce S, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, Didonato JA, Lusis AJ, and Hazen SL (2011). Gut flora metabolism of phophatidylcholine promotes cardivovascular disease. Nature 472(7341): 57–63. doi: 10.1038/nature09922
- Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, and Hazen SL (2014). Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35: 904–910. doi: 10.1093/eurheartj/ehu002
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, and Rey FE (2017). Gut microbiome alterations in Alzheimer's disease. Nat Sci Reports 7(13537): 1–11. doi: 10.1038/s41598-017-13601-y
ACKNOWLEDGMENTS
We acknowledge the following fund-ing sources: NIH/NIDDK K01 DK103952 (JCA) and NIH/NIGMS T32 GM122741 (TNT).
COPYRIGHT
© 2019

The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging by Tibbs et al. is licensed under a Creative Commons Attribution 4.0 International License.