Reviews:
Microbial Cell, Vol. 10, No. 5, pp. 103 - 116; doi: 10.15698/mic2023.05.796
Infinity war: Trichomonas vaginalis and interactions with host immune response
1 Grupo de Pesquisa em Tricomonas, Faculdade de Farmácia e Centro de Biotecnologia, Universidade Federal do Rio Grande do Sul, Avenida Ipiranga, 2752, Porto Alegre, 90610-000, Rio Grande do Sul, Brazil.
Keywords: trichomoniasis, Trichomonas vaginalis, immune response, inflammation.
Received originally: 28/10/2022 Received in revised form: 07/03/2023
Accepted: 21/03/2023
Published: 30/03/2023
Correspondence:
Tiana Tasca, Avenida Ipiranga, 2752. 90610-000. Porto Alegre, Rio Grande do Sul, Brazil; Tel: +555133085325; tiana.tasca@ufrgs.br
Conflict of interest statement: Authors declare no conflict of interest.
Please cite this article as: Giulia Bongiorni Galego and Tiana Tasca (2023). Infinity war: Trichomonas vaginalis and interactions with host immune response. Microbial Cell 10(5): 103-116. doi: 10.15698/mic2023.05.796
Abstract
Trichomonas vaginalis is the pathological agent of human trichomoniasis. The incidence is 156 million cases worldwide. Due to the increasing resistance of isolates to approved drugs and clinical complications that include increased risk in the acquisition and transmission of HIV, cervical and prostate cancer, and adverse outcomes during pregnancy, increasing our understanding of the pathogen’s interaction with the host immune response is essential. Production of cytokines and cells of innate immunity: Neutrophils and macrophages are the main cells involved in the fight against the parasite, while IL-8, IL-6 and TNF-α are the most produced cytokines in response to this infection. Clinical complications: T. vaginalis increases the acquisition of HIV, stimulates the invasiveness and growth of prostate cells, and generates an inflammatory environment that may lead to preterm birth. Endosymbiosis: Mycoplasma hominis increased cytotoxicity, growth, and survival rate of the parasite. Purinergic signaling: NTPD-ases and ecto-5’-nucleotidase helps in parasite survival by modulating the nucleotides levels in the microenvironment. Antibodies: IgG was detected in serum samples of rodents infected with isolates from symptomatic patients as well as patients with symptoms. However, antibody production does not protect against a reinfection. Vaccine candidate targets: The transient receptor potential- like channel of T. vaginalis (TvTRPV), cysteine peptidase, and α-actinin are currently cited as candidate targets for vaccine development. In this context, the understanding of mechanisms involved in the host-T. vaginalis interaction that elicit the immune response may contribute to the development of new targets to combat trichomoniasis.
INTRODUCTION
The unicellular protozoan Trichomonas vaginalis is the etiologic agent of human trichomoniasis, the most common non-viral sexually transmitted infection (STI) in the world. The figures of incidence are higher than Chlamydia infection, gonorrhea, and syphilis. In 2016, the World Health Organization (WHO) estimated an incidence of 156 million cases worldwide, while in the United States of America, according to the Center for Disease Control and Prevention (CDC), there was an incidence of 6.9 million in 2018 with direct medical costs of $144 million [1][2]. Both men and women can be asymptomatic. In women, when symptoms occur, they manifest by vaginal discharge, itching, dysuria, and abdominal pain. In men, the most common symptoms are prostatitis, decreased sperm motility, and epididymitis [3]. In addition, several studies have shown that T. vaginalis infection may increase the acquisition and transmission of HIV in women [3][4]. Co-infection with Chlamydia, gonorrhea, syphilis, and herpes simplex virus type 1 and 2 have also been described [3].
–
Currently, there are three drugs approved by the Food and Drug Administration (FDA) against trichomoniasis: metronidazole, tinidazole, and secnidazole, representatives of the 5-nitroimidazole class [1][5]. However, the rate (11 to 28%) of metronidazole-resistant isolates is an increasing problem [5]. The first metronidazole-resistant isolate was detected in 1962, two years after its introduction [6]. Likewise, the newest therapeutic alternative, secnidazole, has already resistant isolates [5]. Furthermore, the tests with the highest sensitivity and specificity for detection of T. vaginalis, known as nucleic acid amplification tests (NAATs), are not routine in most of clinical laboratories. This, together with the fact that trichomoniasis is not a reportable STI, may contribute to underdiagnosis and consequently underestimation of the number of cases [7].
–
In this context, the understanding of the mechanisms involved in the interaction between T. vaginalis and the host immune response may contribute to the development of new targets to fight the parasite. The search strategy here was based on the combination of T. vaginalis or trichomoniasis with different keywords: immune, immunity, macrophages, neutrophils, immune response, innate immunity, adaptive immunity, purinergic signaling, pregnancy, antibody, Mycoplasma hominis, vaccine, and HIV. Only articles indexed in the PubMed Central® database, in English or Portuguese, published between 2000 and 2022 were included in this review in order to summarize the updated current data on the trichomoniasis immune response field.
THE AVENGERS: THE CELLS OF THE HOST IMMUNE SYSTEM AND CYTOKINE PRODUCTION
During an infection, host defense begins with innate immunity mediated by myeloid cells, natural killer cells, innate lymphoid cells, the complement system, and defensins [8]. Neutrophils are polymorphonuclear cells (PMNs), one of the most important members of the immune response. They can be recruited by pattern recognition receptors present on endothelial cells or inflammatory mediators released by resident leukocytes in tissues [9].
–
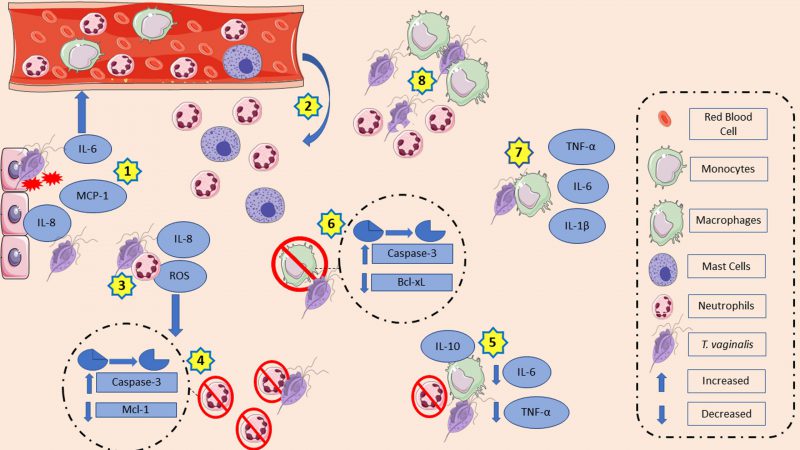
Recently, analyses of T. vaginalis lipophosphoglycan (LPG) concluded that the glycan core lacks significant amounts of phosphate compared to other LPGs, such as Leishmania major LPG. Thus, changing the term T. vaginalis LPG for T. vaginalis lipoglycan (TvLG) is suggested. The poly-LacNAc/LNB chains present in TvLG seem to be required for the parasite to bind to host ectocervical cells [10]. Human ectocervical and endocervical cells, as well as human vaginal epithelial cells (VECs) produced IL-8 and macrophage inflammatory protein (MIP)-3α in response to TvLG. IL-8 is associated with neutrophil migration to the infection site, while MIP-3α is a chemokine that attracts immune cells and is also required for the maturation of dendritic cells [11]. VECs, in the presence of live T. vaginalis, increased IL-6 and IL-8 production, as well as monocyte chemoattractant protein-1 (MCP-1) mRNA expression. As a consequence, the media collected from culture supernatants of VECs in contact with T. vaginalis (TCM) increased the migratory ability of neutrophils and mast cells. In turn, supernatants of mast cells incubated with TCM also stimulated neutrophil migration [12]. Interestingly, the species of bacterial vaginosis (BV) prevalent at the site of infection at the time of parasite inoculation seem to be related to the immunological effects exerted by the parasite. For example, Prevotella bivia, a common agent of bacterial vaginosis, increased IL-8 production induced by the protozoan, whereas MIP-3α induction was lower when both microorganisms were together vs. P. bivia alone. On the other hand, T. vaginalis decreased Lactobacillus species typically present in the vaginal microbiota of women without BV [13]. IL-8 is a proinflammatory cytokine which can regulate the activation, migration, and degranulation of neutrophils. It is released with inflammatory stimulations. Adhesion or contact between the parasite and neutrophils is essential for the production of this cytokine; the membrane integrity of the protozoan plays a role in this too since lysates of trichomonads and T. vaginalis secretory products (TvSP) are not able to produce the same amount of IL-8. The pathways that likely contribute to promoting increased cytokine transcription are the nuclear kappa B (NF-kB) and mitogen-activated protein kinase (MAPK) [14]. Although secretory products do not stimulate neutrophils in the same way as live trichomonads do, they also contribute to IL-8 production possibly due to lipid mediator leukotrienes (LTB4) in their composition. It is hypothesized that LTB4 interacts with high affinity (BLT1) and low affinity (BLT2) LTB4 receptors present on neutrophils and activate the NF-kB and cAMP response element-binding (CREB) pathway. Both appear to be involved in cytokine production [15][16]. Human vaginal and cervical epithelial cells express and secrete galectin-1 and galectin-3, which play opposite roles during trichomoniasis and act as pathogen recognition receptors for TvLG. In this context, galectin-1 has immunosuppressive properties, while galectin-3 has immunostimulant ones. T. vaginalis decreased galectin-3 levels, which decreased the LG pattern recognition. As a result, there is an expected decrease in the recruitment of macrophages and neutrophils as this galectin mediates infection clearance. Meanwhile, the parasite can benefit from the high binding affinity of TvLG to galectin-1, which decreases chemokines levels, such as IL-8 and MIP-3α. IL-8 is expected to reduce the recruitment of neutrophils and macrophages, while MIP-3α is expected to reduce dendritic cells [17].
–
The pathogen-neutrophil interaction goes beyond cytokine production. This in vitro direct contact decreases the myeloid cell leukemia 1 (Mcl-1) expression, an anti-apoptotic protein previously associated with PMNs survival, and increases caspase-3 expression, a protein related to spontaneous apoptosis in neutrophils that triggers the acceleration of the apoptosis process [18]. Interestingly, while live trophozoites enhanced the rate of neutrophil apoptosis, trichomonads lysate reduced it. T. vaginalis lysate co-incubation with PMNs maintained Mcl-1 expression for a longer time than the control group did. In addition, there was a reduction in caspase-3 activation after 15 and 25 hours of incubation, while caspase-3 cleavage by the untreated group occurred after 15 hours of incubation [19]. Reactive oxygen species (ROS) function as signaling messengers. Increased ROS induces mitogen activated protein kinases, protein kinase C, and stimulation of cell proliferation or death. In neutrophils, ROS production aids in the elimination of ingested pathogens. In response to the presence of trichomonads, neutrophils increase intracellular ROS via the NADPH oxidase system, resulting in a possible caspase-3 activation. Activation of caspase-3 has been suggested as part of the apoptosis pathway induced by T. vaginalis in human neutrophils [20]. Trichomonad lysate, secretory product, and membrane components stimulated superoxide anion production by neutrophils as a possible host defense mechanism. In contrast, this production was lower due to protozoan peptidase inhibition [21]. Inhibition of adenosine deaminase, the enzyme that converts adenosine into inosine, leads to increased adenosine bioavailability. As a result, there are low ROS and IL-8 levels due to the anti-inflammatory effects of adenosine [22]. Meanwhile, nitric oxide (NO) is also involved in immune defense mechanisms, and its production by neutrophils has been reported. In the presence of inosine and adenosine, a nucleoside with an immunosuppressive effect, there is a decrease in NO generation by PMNs, suggesting a contribution to the establishment of infection [23]. Furthermore, the parasite could degrade NO in anaerobic conditions probably due to the action of A-type flavoprotein, an NADH-dependent enzyme that responds to NO species exposure. During nutrient deprivation, overexpressed T. vaginalis macrophage migration inhibitory factor (TvMIF) was detected, which is a human cytokine homolog that mimics the HuMIF (human macrophage migration inhibitory factor). It has been proposed that this overexpression of TvMIF can inhibit ROS production and prevent ROS-induced apoptosis [24][25]. Interestingly, reactive nitrogen intermediates (RNI) may be associated with establishing an infection. In order to eliminate an infection, macrophages can produce RNI in response to increased production of proinflammatory cytokines. Isolates of T. vaginalis from symptomatic patients inoculated into mice generated higher levels of RNI in vaginal tissue than isolates from asymptomatic ones. This was a possible way to decrease protozoa population in vaginal washings. In contrast, leukocytes showed a higher RNI concentration and nitric oxide isoform (iNOS) protein band intensity in the asymptomatic group. Meanwhile, RNI in vaginal washes and plasma were higher in the asymptomatic group [26][27].
–
VECs and human prostatic epithelial cells in contact with T. vaginalis or rTvα-actinin 2 increased the production of IL-10, IL-12, IL-6, and tumor necrosis factor alpha (TNF-α). Meanwhile, co-incubation of bone marrow-derived dendritic cells (BMDCs) with TvLG increased the dendritic cell activation markers CD80 and CD86 and the major histocompatibility complex (MHC), opposed to MHC-II expression, which decreased after rTvα-actinin, an immunogenic protein found in the parasite’s secretome. Likewise, BMDCs co-incubated with TvLG and later with TvSP decreased the expression of MHC-II. Nevertheless, when TvSP was present, even with prior TvLG stimulation, IL-10 production increased and IL-12 decreased. TvLG-stimulated BMDCs were subsequently treated with rTvα-actinin 2. Inducible regulatory T cells (iTreg) were incubated with these cells. As a result, iTregs showed a greater release of IL-10, TGF-β, and IFN-γ. These data suggest that rTvα-actinin 2 may act as an immunomodulator [28][29].
–
Macrophages are also members of the innate immune system and play a crucial role in the host defense against pathogens. These cells can secrete cytokines, defensins, ROS, and NO [30]. Thus, the influence of macrophages during T. vaginalis infection was also explored. In vitro, human macrophages in the presence of apoptotic neutrophils induced by T. vaginalis increased IL-10 production and decreased IL-6 and TNF-α [31]. However, in the presence of lysate, opsonized or live trichomonads, human macrophages increased IL-1β, IL-6, and TNF-α levels. The latter may be dependent on NF-kB activation by NO [32]. T. vaginalis adhesion to macrophages is associated with cytokine production. In the first minutes of the adhesion process, there was degradation of IkB-α, likely leading to a reduction in NF-kB activation since IkB-α prevents the binding of NF-kB. However, in a longer adhesion time (eight hours), there was inhibition of NF-kB and consequently a reduction in the TNF-α and IL-12 production. This suggests a possible pathway that parasites use to avoid the generation of cytokine [33]. In mouse macrophages, cytokine production associated with the toll-like receptor (TLR)-2. The presence of the parasite causes phosphorylation of NF-kB p65, p38, and ERK via TLR-2. In addition, p38 and ERK activation resulted in an upregulated expression of the proinflammatory cytokines IL-6, TNF-α and INF-γ, while NF-kB translocation to nucleus via TLR-2 is suggested as an activation pathway. NF-kB activation has been associated with the regulation of NO production [34].
–
During murine macrophage apoptosis induced by co-incubation of T. vaginalis for 8 h, there was downregulation of Bcl-xL, an anti-apoptotic protein. Downregulation of Bcl-xL expression is associated with caspase-3 activation, as caspase-3 is involved in the downregulation of Bcl-xL. On the other hand, the overexpression of Bcl-2 did not protect macrophages from apoptosis nor affected cytochrome c release or Bax and caspase-3 activation [35]. The release of cytochrome c by mitochondria activated the caspase-3, which, in turn, activated the p38 MAPK pathway and resulted in host cell death [36]. Another way for T. vaginalis to induce macrophage cell death is via pyroptosis due to NLRP3 inflammasome activation. This activation needs two signals: the priming step and the activation step. In the priming step, NF-kB is activated and consequently the genes encoding pro-IL1β and NLRP3 increased transcription levels. The activation step is initiated by the host cells danger-associated molecular patterns (DAMPS), such as ATP, and pathogen-associated molecular patterns (PAMPs), such as bacterial surface proteins. Human macrophages in contact with the parasite lead to NLRP3 inflammasome activation via P2X7 receptor, which interacts with extracellular ATP. Once activated, there is an increase in capase-1 activity, leading to bioactive IL-1β generation through the processing of pro-IL-1β. As a consequence of inflammasome activation, macrophages are killed by pyroptotic cell death [37].
–
Finally, immune system cells also have their ways of preventing the establishment of T. vaginalis infection. Neutrophils have two ways to eliminate pathogens: phagocytosis or neutrophil extracellular traps (NET). In NET, a rearrangement of the cytoskeleton occurs, by which nuclear DNA is released into the extracellular DNA. Thus NET pathogens are entrapped and killed. The death caused by NET is known as NETosis. In mice, trichomonads stimulated neutrophils to produce NETs via ROS generation and ERK1/2 and p38 MAPK signaling pathways [38]. After the in vitro co-incubation of T. vaginalis and human neutrophils under NET-forming conditions, the parasite grew 35.2%. In addition, 32.9% of trophozoites remained intact after this contact. This data showed the involvement of NETs, as suggested by [39]. In vaginal discharges, NETs may have different phenotypes with T. vaginalis stimulation [40]. Nevertheless, a few years earlier, the death of the parasite was shown to be independent of NETosis. The contact between PMNs and T. vaginalis was necessary to kill the parasite via trogocytosis, a killing mechanism by which neutrophils “nibble on” pathogens. The participation of serine peptidases and components present in human serum were important in this process. A pre-treatment with AEBSF, a serine peptidase inhibitor, decreased parasite death by more than 85%. Meanwhile, in the absence of human serum, protozoan death by neutrophils decreased, suggesting that opsonization took a part in trogocytosis [41]. Likewise, human monocytic cells used extracellular traps (ET) to capture T. vaginalis using the same pathway and formation as proposed above. Furthermore, in this context, both live and dead parasites could induce ET formation [42].
–
T. vaginalis has been shown to stimulate vaginal, ectocervical, and endocervical epithelial cells to release cytokines that can recruit and activate innate immune cells such as neutrophils, macrophages, and mast cells of the infection site. At the infection site, the parasite has a complex machinery that modulates ROS, cytokine, and chemokine production and induces apoptosis of the host’s immune cells. It also interferes in the maturation of antigen-presenting cells, such as dendritic cells. All together, these mechanisms allow pathogen survival and infection establishment. Fig. 1 summarizes the main mechanisms discussed in this topic.
–
WAR CONSEQUENCES: ASSOCIATION TO HIV, OUTCOMES DURING PREGNANCY, AND PROSTATE CANCER
The presence of T. vaginalis has been associated with several clinical complications such as prostate cancer, preterm birth, and increased acquisition and transmission of HIV [43]. Due to the importance of this subject, this section focuses on the main immunological mechanisms described in the last two decades, exceptionally the paragraph “HIV association,” which included articles outside this time period due to its high relevance.
–
Infection with T. vaginalis increased the risk of acquiring HIV by 1.5 times compared to uninfected individuals. In addition, women with HIV showed a trichomoniasis prevalence of 17-20%, with high rates of repeated infection (22.7% in about 16 months) [44][45]. Some mechanisms may explain this association. It is suggested that T. vaginalis promotes an alteration in the vaginal microbiota by reducing lactobacilli producers of H2O2, causing a change in pH and favoring BV. In turn, BV enhanced pro-inflammatory cytokines that disrupted the mucosal barrier, while trichomonads disrupt the integrity of the cell monolayer by themselves. Both mechanisms may facilitate HIV-1 passage. The immune response activation due to the T. vaginalis infection observed in in vitro studies is expected to lead to recruitment of CD4 lymphocytes, the target cells of HIV. Peripheral blood mononuclear cells (PBMCs) infected with HIV-1 in contact with the parasite released TNF-α, which is partially involved with enhancing viral replication [46][47][48][49].
–
Women with positive results for T. vaginalis-antigen test showed lower levels of secretory leukocyte protease inhibitor (SLPI). In vitro, the parasite’s cysteine peptidases cleave the SLPI, a defense mechanism cited against acquisition of HIV infection [50]. However, a cross-sectional study found no association between the presence of T. vaginalis and the enhanced expression or activation of CD4+ cells of the HLADR+CD38+ phenotypes, which is in disagreement with the aforementioned studies that suggested it as one of the pathways involved in facilitating HIV infection [51].
–
In pregnant women, the parasite leads to low birth weight and preterm delivery. The molecular mechanisms for this association are still to be identified [52]. In a population of pregnant women with less than 20 weeks of gestation, co-infection with T. vaginalis and BV increased IL-1β and IL-8. It has been suggested that high levels of IL-1 β in response to colonization of anaerobic Gram-negative bacteria may possibly be associated with preterm delivery between 18-22 weeks of gestation [53][54]. In agreement with these findings, a cohort study evaluated 65 asymptomatic pregnant patients infected with T. vaginalis. The concentration of cervical IL-8 and neutrophil defensins in vaginal fluid were higher in women with asymptomatic trichomoniasis than in the uninfected group, confirming cytokine release and neutrophil activation during trichomoniasis [55]. Furthermore, T. vaginalis infection during pregnancy increased the serum concentration of granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF is predicted to stimulate the production of immune cells such as macrophages, dendritic cells, and granulocytes. Serum C-reactive protein (CRP) levels were also high in T. vaginalis-infected women. Both findings indicate a systemic inflammatory response [56].
–
The symbiosis between T. vaginalis and M. hominis may play a role in the clinical worsening observed in trichomoniasis during pregnancy. In vitro studies have found that after treatment with metronidazole, large amounts of bacteria were released in the extracellular environment. Once they are free in this environment, the bacteria can infect human cells [57]. Another study found a prevalence of 9.5% of this bacterial microorganism. It is cited as one of the causes of intra-amniotic infection, which may result in preterm birth [58].
–
The association between T. vaginalis and prostate cancer is debatable. While some studies have shown this mechanism to be involved in signaling tumor-like cells, other studies have not found epidemiological evidence [59][60]. Due to the focus of this review, the topic below will discuss the immunological mechanisms described for this association. Epithelial mesenchymal transition (EMT) is the transformation of epithelial cells into stromal cells, contributing to tumor progression. In this context, human prostate epithelial cells co-incubated with live T. vaginalis increased CCL2 mRNA, but not its protein levels, while CXCL8, IL-1β, and IL-6 increased both protein and mRNA levels. This interaction induced EMT via NF-kB and JAK/STAT signal pathway. Meanwhile, an increase in H2O2 generation via the NADPH oxidase system has suggested an involvement in the regulation of ERK, while NF-kB inhibitors decreased IL-1β production, suggesting a role of NF-kB in IL-1β production. Some proposed that IL-1β and CXCL8 can lead to monocyte migration. IL-6, via the IL-6R/JAK2/STAT3 signaling pathway, stimulated polarization of M2 macrophages, which accumulate in prostate tumor tissue and contribute to the proliferation of prostate cancer cells [61][62][63]. In vivo, prostate cancer cells from PC3 lineage cells with T. vaginalis-contained medium (TCM) injected into a PC3 xenograft mouse model resulted in an increase in tumor weight and volume and in EMT-related markers, proliferating cell nuclear antigen (PCNA) and cyclin D1, which are proliferative signal molecules [64]. Interestingly, adipocytes can also increase prostate cancer development. Co-incubation of mouse prostate cancer cells with T. vaginalis made adipocytes to increase production of IL-4, CCL2, and IL-6, as well as mRNA levels of CCL2, IL-4, and IL-13. In turn, IL-4 and IL-13 stimulated macrophage migration and polarization to M2 macrophages [65]. Prostate stromal cells infected with trichomonads recruited neutrophils and monocytes by CXCL8, CCL2 released via activation of TLR4, ROS, MAPK, and NF-kB [66]. Benign prostatic hyperplasia (BPH) cells stimulated by the parasite can in turn stimulate proliferation of prostate stromal cells. BPH cells in contact with trichomonads (TCM) produced cytokines and chemokines (CXCL8, CCL2, IL-1β, and IL-6), which led to mast and monocytes cell migration and activated mast cells. In turn, prostate stromal cells in contact with activated mast cells stimulated by the TCM supernatant (M-TCM) increased CXCL8 and CCL2 levels. These cytokines seem to be involved in prostate stromal cell proliferation via CXCL8-CXCR1 and CCL2-CCR2 signaling. Furthermore, prostate stromal cells showed an increase in cyclin D1 and B-cell lymphoma-2 (Bcl-2) expression in response to incubation with supernatant of M-TCM [67][68]. BPH cells stimulated with T. vaginalis were subsequently incubated with mast cells. This supernatant was then put in contact with prostate stromal cells. The result was that this supernatant stimulated BPH epithelial cells proliferation. IL-6 seems to be involved in the proliferation of these cells and in EMT induction [69]. Mouse cells showed the same behavior. In vitro, media collected from mouse prostate cancer (PCa) cells in contact with T. vaginalis stimulated an increased migratory ability of macrophages. This was expected to occur through the increased production of CCL2 and CXCL1 detected in co-incubation media. Macrophages exposed to PCa T. vaginalis media showed an increased expression of CCL2, IL-6, and TNF-α at mRNA level. Conditioned media from T. vaginalis-infected PCa cells incubated with macrophages stimulated PCa cell proliferation and invasiveness. Furthermore, these cells expressed the cytokine receptors GP130, CCR2, and CXCR2, expected to be positively involved with the proliferation and invasiveness of these cells [70]. In vivo, when the mouse prostate was injected with a mixture of trichomonads and PCa cells, there was an increase in cyclin D1 and N-cadherin expression, opposed to a decrease in E-cadherin expression. In addition, this group showed a larger volume and weight of prostate cancer compared to the group injected only with PCa cells [71].
–
Finally, TvMIF production by T. vaginalis possibly contributes to an inflammatory environment through the interaction with monocytes and CD74 receptors, the HuMIF receptor. TvMIF binds to the CD74 receptor and leads to downstream ERK1/2, Akt, and BAD activation involved with increased cell proliferation and inflammation [72]. Interestingly, TvMIF is frequently found in patients with positive rather than negative infection, suggesting there is a TvMIF release in the extracellular environment in vivo. In addition, it has been detected especially in men, either because of the stronger immune response of men against TvMIF or because there is a greater release by the parasite in men than in women. TvMIF release possibly contributes to increased risk of human prostate cancer due to increased cell proliferation and invasion [72].
–
The long permanence of T. vaginalis may cause several clinical complications. Three of them are highlighted: the increase in HIV transmission and acquisition due to the disruption of the mucosal barrier, reduction of SLPI, and recruitment of CD4+T cells; adverse pregnancy outcomes caused by a systemic inflammatory response with cytokine production, neutrophil migration, and M. hominis release into the extracellular environment; and prostate cancer attributed to a sequence of inflammatory mechanisms that increased the invasiveness and growth of prostate cells, creating a perfect microenvironment for the establishment of cancer.
AN EXTRA HELP OF M. HOMINIS AGAINST HOST IMMUNE RESPONSE
The endosymbiosis between M. hominis and T. vaginalis has gained prominence in recent years. Some articles showed that the bacteria can survive within the parasite’s cytoplasmic vacuoles and their presence can influence T. vaginalis biochemical pathways and immunomodulation. It is predicted to contribute to tumorigenesis and adverse pregnancy outcomes. Thus, this association may affect the immune response, survival, and outcomes observed during trichomoniasis [73]
–
This interaction increased the mycoplasma drug resistance and protected M. hominis from antibiotics. The 5-Bromodeoxyuridine (5-BrdU) incorporation assay suggested that the bacterial DNA is incorporated into trichomonad DNA. The assay is based on the lack of mitochondria in the parasite and therefore absence of extranuclear DNA. Thus, 5-BrdU incorporation in a location different than the nucleus indicates replication activity from another organism. Cultures of T. vaginalis with M. hominis were submitted to 5-BrdU assay. As a result, 5-BrdU incorporation was detected, which indicated DNA synthesis by the bacteria inside the parasite. The bacteria identity was confirmed using anti-M. hominis antibodies. Mycoplasma DNA incorporation into T. vaginalis DNA could possibly affect PCR-based analytical methods, a golden pattern of trichomoniasis laboratorial diagnosis [74][75][76]. On the other hand, the effects on trichomonads’ metronidazole resistance are under debate. Some authors argue that T. vaginalis resistance to metronidazole cannot be attributed to the presence of M. hominis [77][78][79][80], while others speculate that bacterial presence plays a role in modulating enzymatic expression levels related to resistance, such as pyruvate ferredoxin oxidoreductase (PFOR), and demonstrates that seven of eight resistant isolates were M. hominis-positive [81][82]. Furthermore, it is possible that this endosymbiosis may actually reduce resistance to metronidazole, making the isolate more susceptible and suggesting that downregulation of PFOR is not necessarily related to drug resistance [83].
–
The difference among T. vaginalis isolates appears to be related with the number of infecting bacteria, although this endosymbiosis was not observed in all T. vaginalis isolates [84]. Interestingly, in vitro Mycoplasma-positive T. vaginalis isolates can infect both Mycoplasma-free T. vaginalis isolates and human cells, while a single T. vaginalis isolate can be infected by two mycoplasma species [83][84]. Recent studies have shown that T. vaginalis can be infected not only by M. hominis, but also by Candidatus Mycoplasma girerdii, previously known as Mnola, which showed a 78% similarity with M. hominis and 85% with Mycoplasma genitalium. In addition, the hypothesis that the protozoan may have symbiosis with other bacteria, such as Ureaplasma spp., needs further explanation [85][86]. In the presence of any Mycoplasma species, the parasite showed an upregulation of hemolysis and cytoadherence in epithelial cells, indicating a bacterial influence on the virulence observed in T. vaginalis isolates [87].
–
M. hominis and T. vaginalis also affect the immune response. The presence of both microorganisms in symbiosis stimulated IL-8, TNF-α, IL-1β, and IL-23 production by human macrophages and activated the NF-kB pathway, which seems be involved in this cytokine production [88]. In addition, the bacteria may influence the growth rate of T. vaginalis, being ∼20% faster in the presence of the symbiont. Furthermore, a competition between macrophages and bacteria for arginine-free uptake leads to downregulation of NO production by macrophages [89]. Nevertheless, this combination potentiated the cytopathic effects on epithelial cells with low viability and increased in the intercellular spaces, suggesting an increase in virulence since there was a higher rate of amoeboid transformation and phagocytic activity. However, there was no impact on the leukotoxic effects caused by T. vaginalis [90][91]. Interestingly, PBMCs in contact with small extracellular vesicles from isolates containing T. vaginalis virus inhibited the IL-8 response to the signaling of Mycoplasma-derived macrophage-activating lipopeptide-2 (MALP-2), a mycoplasma protein, thus reducing the immune surveillance against bacteria [92].
–
T. vaginalis and Mycoplasma species have a beneficial relationship for both parties. For the bacteria, the parasite works as a “trojan horse,” with extra protection against immune defense and antibiotics in the extracellular environment; for the parasite, there is an increase in growth rate, virulence, and modulation of immune response, allowing its survival under stress conditions. Further studies are needed on the possibility of T. vaginalis carrying other microorganisms, such as Ureaplasma spp.
IMMUNOMODULATORY WEAPONS: T. VAGINALIS AND PURINERGIC SIGNALING AND CD59 SEQUESTRATION
T. vaginalis has enzymes known as ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) and ecto-5′-nucleotidase (E-5N). They are responsible for a cascade that hydrolyzes extracellular nucleotides from tri-, di-, and monophosphate nucleotides to the nucleosides, such as ATP, ADP, and AMP to adenosine (ADO). Extracellular nucleosides not only affect the infection establishment, but also are essential for parasite survival since T. vaginalis cannot synthetize de novo purine and pyrimidine rings, requiring an uptake from the host via salvage pathway. ADO showed anti-inflammatory properties, which are important in the context of establishing an infection. However, studies have shown that some T. vaginalis isolates are deficient in AMP hydrolysis activity [93]. During human infection, this may result in leukocyte infiltration due to low adenosine levels, leading to the acute symptoms observed in patients infected with those isolates [93]. Furthermore, adenosine interacts and activates A2A receptors present in immune cells such as neutrophils, macrophages, and monocytes. Meanwhile, ATP is released during cell damage, plays a role in cytotoxic mechanisms, and stimulates chemotaxis of neutrophils and mast cells, as well as increases cytokines with proinflammatory properties [94]. Interestingly, adenosine nucleotides are preferable for uptake by the parasite than guanosine [95]. Under serum restriction conditions, adenosine deaminase enzymatic activity does not change, while E-5’N had an increased gene expression; it acts as a compensatory pathway, increasing adenosine levels. Under low iron availability conditions, adenosine deaminase decreases its activity, which returns to normal conditions when there is exposure to iron sources [96][97]. Nucleotides also influence virulence factors. A very cytotoxic isolate had its injurious effects potentiated in the presence of ATP accumulation, but when adenosine deaminase, responsible for the conversion of adenosine into inosine, was inhibited by EHNA, the increased availability of extracellular adenosine protected cells against these effects [98]. These findings shed light on the potential of inhibiting ecto-nucleotidases. Lycorine and candimine, alkaloids from Amaryllidaceae plants, were tested as compounds targeting T. vaginalis enzymes. There was an inhibition of NTPDase and ecto-5′-nucleotidase activities after 24 h, suggesting a new way to affect the parasite-host interaction. Lycorine also increased ROS production by neutrophils, which can be an adjuvant in the clearance process against the parasite [99][100].
–
In addition to the aforementioned enzymes, T. vaginalis can also use the sequestration of CD59 from different cells, including erythrocytes, as an immune evasion strategy. CD59 is a human protectin that acts as a complement lysis-restricting factor. Therefore, its sequestration partially increases the parasite protection against complement-mediated lysis [101].
–
In conclusion, NTPDases, E-5’N, and adenosine deaminase provide the parasites with enzymatic mechanisms to modulate the inflammatory environment, contributing to parasite survival. In the same context, the sequestration of CD59 is also one of the ways to evade immune response, helping to avoid targeting by the complement system.
HOPE IN THE MIDST OF WAR: ANTIBODY PRODUCTION DURING TRICHOMONIASIS
During T. vaginalis infection, there is antibody production. Although they are not protective against reinfection, they may be associated with the presence of symptoms or the parasite itself. In this context, some authors have tried to find an association between certain antibodies and symptoms.
–
Using the mouse model, a study evaluated the circulating antibodies during infection caused by T. vaginalis. IgA was present in vaginal washes and serum in greater levels in mice infected with T. vaginalis isolates from asymptomatic patients. Also, IL-2, CD3+ T cells, CD4+ T cells, and NK cells were also with higher levels in mice infected with T. vaginalis isolates from asymptomatic vs. symptomatic patients [102]. Fourteen days after infection, IgG, IgG1, and IgM levels were higher in serum samples and vaginal washes of rodents infected with isolates from symptomatic patients than in isolates from asymptomatic rodents [103]. In agreement, when antibodies in the serum of symptomatic patients were analyzed, IgG and IgM were detected in 66.6% and 33.3% of patients, respectively (400 dilutions). IgA was detected in 100% of cases (>400 dilutions). In vaginal washes, IgM was not detected in symptomatic patients, while IgG was detected in all patients tested (≥40 dilutions) [104].
–
Antibody production is also associated with protection to adverse outcomes of pregnancy (AOP) and therapy success. Pregnant women positive for T. vaginalis with no AOP had a higher IgG anti-TvLG in vaginal secretion than the group with AOP [105]. After effective therapy with metronidazole, a low circulating IgG anti-T. vaginalis was detected, suggesting that there was no longer antigenic stimulation. However, in few patients whose treatment failed, reinfection or infection by resistant isolates led to the detection of antibody, which can be associated with a chronic infection [106].
–
In conclusion, IgG was found in serum samples of rodents infected with isolates from symptomatic patients and patients with no symptoms. However, antibody production is not likely protective. The association between symptoms and the presence of antibodies remains under investigation.
THE INFINITY STONES: VACCINE CANDIDATE TARGETS
Some proteins of T. vaginalis play an essential role in the establishment of infection and in the parasite-host relationship. They are, therefore, promising vaccine candidate targets.
–
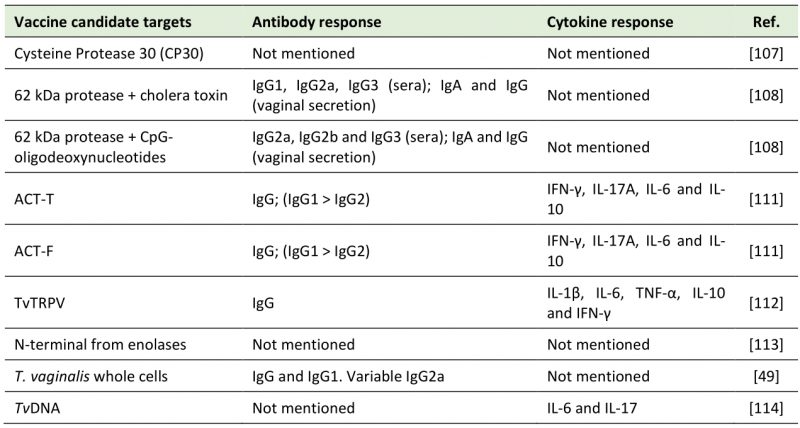
Cysteine protease (CP) 30 was found in all 20 fresh isolates from symptomatic and 20 asymptomatic patients investigated in India. The intensity band of CP30 and the basal cytoadherence to vaginal epithelial cells were higher in isolates from symptomatic than from asymptomatic patients. Although the antibodies anti-CP30 inhibited cytoadherence in asymptomatic and symptomatic isolates, the specific inhibition of cytoadherence was lower in asymptomatic isolates vs. symptomatic ones. Thus, CP30 was suggested as a virulence factor of T. vaginalis [107]. The group of BALB/c mice immunized with intranasal injection of 62 kDa cysteine-proteinase (p-62) plus cholera toxin (CT) increased IgG1, IgG2a, and IgG3 serum circulating levels. In sera of the immunized group of p-62 plus CpG-oligodeoxynucleotides (CpG), there was an increase in IgG2a, IgG2b and IgG3. Vaginal secretion in both groups showed IgG and IgA levels specific for p-62. However, IgA response was higher in p-62 plus CT group [108]. A few years later, T. vaginalis anti-protease monoclonal antibodies (MAbs) named 4D8 and 1A8 were tested against this same protease. The result showed that the antibodies reacted with different epitopes of a repetitive nature. After treatments with pronase, heat and trichloroacetic acid (TCA), reactivity decreased due the epitope alteration, affecting MAbs recognition. In conclusion, 4D8 obtained a better protective activity in murine model than 1A8, which can be explained by the interaction of MAbs with different protein epitopes [109]. In vitro, 4D8 and 1A8 also inhibited cytoadherence to HeLa cells. 62kDa anti-protease MAbs, in the presence of macrophages, increased NO levels. However, there was no effect on cysteine protease of T. vaginalis antigen [110].
–
Two peptides from α-actinin present in the parasite (ACT-F and ACT-T) were used at high subcutaneous dosages for immunization. ACT-T conferred a protection of 100% to mice, while the high dosage of ACT-F only protected 55% of the animals. High amounts of IFN-γ, IL-17A and IL-6 cytokines and barely any IL-2 and IL-4 were detected in both tested groups. In addition, both peptides produced high levels of IgG, specifically IgG1, which was detected at greater concentrations than IgG2 [111]. The transient receptor potential-like channel of T. vaginalis (TvTRPV) was also tested as a vaccine candidate target. At high doses, there was an increased immune response in BALB/c mice with high IgG production in serum. In addition, TvTRPV can be recognized by macrophages, which stimulated the production of IL-1β, IL-6 and TNF-α. CD4+T cells isolated from TvTRPV-immunized mice were incubated with mouse macrophages treated with TvTRPV. As a result, CD4+T cells produced IL-10 and IFN-γ [112]. The latter is expected to potentiate macrophage cytotoxicity against the protozoan, while IL-10 is associated with the initial anti-inflammatory feedback loop [112].
–
A unique N-terminus from enolases, a group of enzymes that participates in glycolysis, was also suggested as a potential target against T. vaginalis. However, in this study, there was no development or test as an immunizing agent [113]. Finally, whole T. vaginalis cells were proposed as an immunization strategy. The mice group that received the vaccine candidate had higher IgG and IgG1 serum total levels than the unimmunized group. The production of IgG2a varied between the groups tested [49]. Similarly, the pretreatment of mice with TvDNA two days before the parasite inoculation resulted in less available trophozoites in vaginal washes and infection signs. Higher levels of IL-6 were observed between four to 14 days post-infection in the pre-treated group compared to the untreated group. There was a higher production of IL-17 throughout the 14 days after infection. However, there were undetectable levels of IL-10 from four to ten days after the infection [114].
–
One of the proposed ways to fight trichomoniasis is immunization. However, due to lack of an adequate animal model for T. vaginalis infection, this becomes an arduous task. Animal models that are used to study trichomoniasis infection present some important limitations. The route of inoculation can be subcutaneous, intraperitoneal, or vaginal. However, the first two are not the site of T. vaginalis infection in the host, therefore they do not accurately mimic the infection in humans. In the intraperitoneal route, successful infection is associated with gross-pathologic changes, ascitic fluid, and mortality [115][116]. Another way to infect mice is through vaginal infection. This model is useful to understand the virulence of the parasite. However, for the infection to occur and be sustained, it is necessary that animals are pretreated with subcutaneous estradiol and intraperitoneal dexamethasone. An analysis of the presence of PMNs indicated that this treatment inhibited the migration of these cells, which are expected to affect the immunological analysis of this animal model [115][117]. Furthermore, pretreatment with bacteria was also tested. A pre-inoculation of mice with Lactobacillus acidophilus increased T. vaginalis infection duration. Interestingly, there was no alteration in the vaginal microbiome of mice after Lactobacillus inoculation. However, prior to L. acidophilus inoculation, a subcutaneous estradiol injection is required. Treatment with estrogen may increase the vaginal antibody response [115][118], which affects the accuracy of antibody analysis, important in vaccine development studies.
–
Table 1 summarizes the vaccine candidate targets and the immune system activation discussed in this topic.
–
Table 1. Vaccine candidate targets against trichomoniasis. |
 |
CONCLUSION
This review highlights parasite strategies to activate and stimulate or evade variated and complex immunological mechanisms related to the symptoms and clinical complications observed here. The symbiosis with M. hominis also requires further studies to understand the extent of this relationship. As a conclusion of this review, the war between T. vaginalis and the host immune system is far from over. Further studies in immunology are necessary to understand the pathogen behavior aiming to discover new targets to fight this infection.
REFERENCES
- National Center for Health Statistics (2021). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm [Accessed 25.10.2022]
- Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 97(8): 548-562P. 10.2471/BLT.18.228486
- Kissinger P (2015). Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 15:307. 10.1186/s12879-015-1055-0
- Mercer F, Johnson PJ (2018). Trichomonas vaginalis: Pathogenesis, Symbiont Interactions, and Host Cell Immune Responses. Trends Parasitol 34(8): 683-693. 10.1016/j.pt.2018.05.006
- Mtshali A, Ngcapu S, Govender K, Sturm AW, Moodley P, Joubert BC (2022). In Vitro Effect of 5-Nitroimidazole Drugs against Trichomonas vaginalis Clinical Isolates. Microbiol Spectr 10(4): e0091222. 10.1128/spectrum.00912-22
- Robinson SC (1962). Trichomonal Vaginitis Resistant to Metranidazole. Can Med Assoc J 86(14): 665. 20327097
- Bruni MP, Freitas da Silveira M, Stauffert D, Bicca GLO, Caetano Dos Santos C, da Rosa Farias NA, Golparian D, Unemo M (2019). Aptima Trichomonas vaginalis assay elucidates significant underdiagnosis of trichomoniasis among women in Brazil according to an observational study. Sex Transm Infect 95(2): 129-132. 10.1136/sextrans-2018-053635
- Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL (2019). Innate and Adaptive Immune Memory: an Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 25(1): 13-26. 10.1016/j.chom.2018.12.006
- Liew PX, Kubes P (2019). The Neutrophil’s Role During Health and Disease. Physiol Rev 99(2): 1223-1248. 10.1152/physrev.00012.2018
- Ryan CM, Mehlert A, Richardson JM, Ferguson MA, Johnson PJ (2011). Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (β1-4/3) N-acetylglucosamine repeats in host cell interaction. J Biol Chem 286(47): 40494-508. 10.1074/jbc.M111.280578
- Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN (2006). Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 74(10): 5773-9. 10.1128/IAI.00631-06
- Han IH, Park SJ, Ahn MH, Ryu JS (2012). Involvement of mast cells in inflammation induced by Trichomonas vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol 34(1): 8-14. 10.1111/j.1365-3024.2011.01338.x
- Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB (2013). The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 89(6): 460-6. 10.1136/sextrans-2013-051052
- Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY (2004). Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun 72(3): 1326-32. 10.1128/IAI.72.3.1326-1332.2004
- Nam YH, Min A, Kim SH, Lee YA, Kim KA, Song KJ, Shin MH (2012). Leukotriene B(4) receptors BLT1 and BLT2 are involved in interleukin-8 production in human neutrophils induced by Trichomonas vaginalis-derived secretory products. Inflamm Res 61(2): 97-102. 10.1007/s00011-011-0425-3
- Nam YH, Min D, Park SJ, Kim KA, Lee YA, Shin MH (2011). NF-κB and CREB are involved in IL-8 production of human neutrophils induced by Trichomonas vaginalis-derived secretory products. Korean J Parasitol 49(3): 291-4. 10.3347/kjp.2011.49.3.291
- Fichorova RN, Yamamoto HS, Fashemi T, Foley E, Ryan S, Beatty N, Dawood H, Hayes GR, St-Pierre G, Sato S, Singh BN (2016). Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and -3 to Modulate Epithelial Immunity. J Biol Chem 291(2): 998-1013. 10.1074/jbc.M115.651497
- Kang JH, Song HO, Ryu JS, Shin MH, Kim JM, Cho YS, Alderete JF, Ahn MH, Min DY (2006). Trichomonas vaginalis promotes apoptosis of human neutrophils by activating caspase-3 and reducing Mcl-1 expression. Parasite Immunol 28(9): 439-46. 10.1111/j.1365-3024.2006.00884.x
- Song HO, Lim YS, Moon SJ, Ahn MH, Ryu JS (2010). Delayed human neutrophil apoptosis by Trichomonas vaginalis lysate. Korean J Parasitol 48(1): 1-7. 10.3347/kjp.2010.48.1.1
- Song HO, Shin MH, Ahn MH, Min DY, Kim YS, Ryu JS (2008). Trichomonas vaginalis: reactive oxygen species mediates caspase-3 dependent apoptosis of human neutrophils. Exp Parasitol 118(1): 59-65. 10.1016/j.exppara.2007.06.010
- Song HO, Ryu JS (2013). Superoxide anion production by human neutrophils activated by Trichomonas vaginalis. Korean J Parasitol 51(4): 479-84. 10.3347/kjp.2013.51.4.479
- Frasson AP, Menezes CB, Goelzer GK, Gnoatto SCB, Garcia SC, Tasca T (2017). Adenosine reduces reactive oxygen species and interleukin-8 production by Trichomonas vaginalis-stimulated neutrophils. Purinergic Signal 13(4): 569-577. 10.1007/s11302-017-9584-1
- Frasson AP, De Carli GA, Bonan CD, Tasca T (2012). Involvement of purinergic signaling on nitric oxide production by neutrophils stimulated with Trichomonas vaginalis. Purinergic Signal 8(1): 1-9. 10.1007/s11302-011-9254-7
- Sarti P, Fiori PL, Forte E, Rappelli P, Teixeira M, Mastronicola D, Sanciu G, Giuffré A, Brunori M (2004). Trichomonas vaginalis degrades nitric oxide and expresses a flavorubredoxin-like protein: a new pathogenic mechanism? Cell Mol Life Sci 61(5): 618-623. 10.1007/s00018-003-3413-8
- Chen YP, Twu O, Johnson PJ (2018). Trichomonas vaginalis Macrophage Migration Inhibitory Factor Mediates Parasite Survival during Nutrient Stress. mBio 9(3): e00910-18. 10.1128/mBio.00910-18
- Malla N, Valadkhani Z, Harjai K, Sharma S, Gupta I (2004). Reactive nitrogen intermediates in experimental trichomoniasis induced with isolates from symptomatic and asymptomatic women. Parasitol Res 94(2): 101-5. 10.1007/s00436-004-1155-z
- Yadav M, Dubey ML, Gupta I, Malla N (2006). Nitric oxide radicals in leucocytes and vaginal washes of Trichomonas vaginalis-infected symptomatic and asymptomatic women. Parasitology 132 (Pt3): 339-43. 10.1017/S0031182005009340
- Lee HY, Kim J, Ryu JS, Park SJ (2017). Trichomonas vaginalis α-Actinin 2 Modulates Host Immune Responses by Inducing Tolerogenic Dendritic Cells via IL-10 Production from Regulatory T Cells. Korean J Parasitol 55(4): 375-384. 10.3347/kjp.2017.55.4.375
- Song MJ, Lee JJ, Nam YH, Kim TG, Chung YW, Kim M, Choi YE, Shin MH, Kim HP (2015). Modulation of dendritic cell function by Trichomonas vaginalis-derived secretory products. BMB Rep 48(2): 103-8. 10.5483/bmbrep.2015.48.2.116
- Franken L, Schiwon M, Kurts C (2016). Macrophages: sentinels and regulators of the immune system. Cell Microbiol 18(4): 475-87. 10.1111/cmi.12580
- Ahn MH, Song HO, Ryu JS (2008). Trichomonas vaginalis-induced neutrophil apoptosis causes anti-inflammatory cytokine production by human monocyte-derived macrophages. Parasite Immunol 30(8): 410-6. 10.1111/j.1365-3024.2008.01037.x
- Han IH, Goo SY, Park SJ, Hwang SJ, Kim YS, Yang MS, Ahn MH, Ryu JS (2009). Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J Parasitol 47(3): 205-12. 10.3347/kjp.2009.47.3.205
- Chang JH, Ryang YS, Morio T, Lee SK, Chang EJ (2004). Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-kappaB activation. Mol Cells 18(2): 177-85. 15528993
- Li L, Li X, Gong P, Zhang X, Yang Z, Yang J, Li J (2018). Trichomonas vaginalis Induces Production of Proinflammatory Cytokines in Mouse Macrophages Through Activation of MAPK and NF-κB Pathways Partially Mediated by TLR2. Front Microbiol 9: 712. 10.3389/fmicb.2018.00712
- Chang JH, Ryang YS, Kim SK, Park JY (2004). Trichomonas vaginalis-induced apoptosis in RAW264.7 cells is regulated through Bcl-xL, but not Bcl-2. Parasite Immunol 26(3):141-50. 10.1111/j.0141-9838.2004.00693.x
- Chang JH, Kim SK, Choi IH, Lee SK, Morio T, Chang EJ (2005). Apoptosis of macrophages induced by Trichomonas vaginalis through the phosphorylation of p38 mitogen-activated protein kinase that locates at downstream of mitochondria-dependent caspase activation. Int J Biochem Cell Biol 38(4): 638-47. 10.1016/j.biocel.2005.11.005
- Riestra AM, Valderrama JA, Patras KA, Booth SD, Quek XY, Tsai CM, Nizet V (2019). Trichomonas vaginalis Induces NLRP3 Inflammasome Activation and Pyroptotic Cell Death in Human Macrophages. J Innate Immun 11(1): 86-98. 10.1159/000493585LPL
- Gao Y, Li X, Zhang N, Zhao P, Li S, Tai L, Li X, Li L, Li J, Zhang X, Sun M, Cao L, Gong P (2021). Trichomonas vaginalis induces extracellular trap release in mouse neutrophils in vitro. Acta Biochim Biophys Sin 53(12): 1736-1738. 10.1093/abbs/gmab139
- Ramírez-Ledesma MG, Romero-Contreras YJ, Rodríguez MC, Reyes-Cortes R, Cuéllar-Mata P, Avila EE. Trichomonas vaginalis triggers neutrophil extracellular traps reducing parasite integrity and growth (2022). Parasitol Res 121(5): 1355-1367. 10.1007/s00436-022-07475-x
- Zambrano F, Melo A, Rivera-Concha R, Schulz M, Uribe P, Fonseca-Salamanca F, Ossa X, Taubert A, Hermosilla C, Sánchez R (2022). High Presence of NETotic Cells and Neutrophil Extracellular Traps in Vaginal Discharges of Women with Vaginitis: An Exploratory Study. Cells 11(20): 3185. 10.3390/cells11203185
- Mercer F, Ng SH, Brown TM, Boatman G, Johnson PJ (2018). Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol 16(2): e2003885. 10.1371/journal.pbio.2003885
- Fei L, Zhengkai W, Weina J, Lili C, Yuhang G, Zhengtao Y, Jianhua L, Biao Y, Xichen Z, Pengtao G (2019). Trichomonas vaginalis triggers the release of THP-1 extracellular traps. Parasitol Res 118(1): 267-274. 10.1007/s00436-018-6139-5
- Menezes CB, Frasson AP, Tasca T (2016). Trichomoniasis – are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microbial Cell 3(9): 404-419. 10.15698/mic2016.09.526
- Masha SC, Cools P, Sanders EJ, Vaneechoutte M, Crucitti T (2019). Trichomonas vaginalis and HIV infection acquisition: a systematic review and meta-analysis. Sex Transm Infect 95(1): 36-42. 10.1136/sextrans-2018-053713
- Van Gerwen OT, Muzny CA (2019). Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res 8: F1000 Faculty Rev-1666. 10.12688/f1000research.19972.1
- Moodley P, Connolly C, Sturm AW (2002). Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J Infect Dis 185(1): 69-73. 10.1086/338027
- Mirmonsef P, Krass L, Landay A, Spear GT (2012). The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr HIV Res 10(3): 202-10. 10.2174/157016212800618165
- Guenthner PC, Secor WE, Dezzutti CS (2005). Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun 73(7): 4155-60. 10.1128/IAI.73.7.4155-4160.2005
- Smith JD, Garber GE (2015). Trichomonas vaginalis infection induces vaginal CD4+ T-cell infiltration in a mouse model: a vaccine strategy to reduce vaginal infection and HIV transmission. J Infect Dis 212(2): 285-93. 10.1093/infdis/jiv036
- Huppert JS, Huang B, Chen C, Dawood HY, Fichorova RN (2013). Clinical evidence for the role of Trichomonas vaginalis in regulation of secretory leukocyte protease inhibitor in the female genital tract. J Infect Dis 207(9): 1462-70. 10.1093/infdis/jit039
- Jarrett OD, Brady KE, Modur SP, Plants J, Landay AL, Ghassemi M, Golub ET, Spear GT, Novak RM (2015). T. vaginalis Infection Is Associated with Increased IL-8 and TNFr1 Levels but with the Absence of CD38 and HLADR Activation in the Cervix of ESN. PLoS One 10(6): e0130146. 10.1371/journal.pone.0130146
- Fichorova RN (2009). Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol 83(1-2): 185-9. 10.1016/j.jri.2009.08.007
- Genc MR, Witkin SS, Delaney ML, Paraskevas LR, Tuomala RE, Norwitz ER, Onderdonk AB (2004). A disproportionate increase in IL-1β over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am J Obstet Gynecol 190(5): 1191-7. 10.1016/j.ajog.2003.11.007
- Cauci S, Culhane JF (2007). Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol 196(2): 133.e1-7. 10.1016/j.ajog.2006.08.033
- Simhan HN, Anderson BL, Krohn MA, Heine RP, Martinez de Tejada B, Landers DV, Hillier SL (2007). Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol 196(1): 59.e1-5. 10.1016/j.ajog.2006.08.035
- Anderson BL, Cosentino LA, Simhan HN, Hillier SL (2007). Systemic immune response to Trichomonas vaginalis infection during pregnancy. Sex Transm Dis 34(6): 392-6. 10.1097/01.olq.0000243618.71908.95
- Thi Trung Thu T, Margarita V, Cocco AR, Marongiu A, Dessì D, Rappelli P, Fiori PL (2018). Trichomonas vaginalis Transports Virulent Mycoplasma hominis and Transmits the Infection to Human Cells after Metronidazole Treatment: A Potential Role in Bacterial Invasion of Fetal Membranes and Amniotic Fluid. J Pregnancy 2018: 5037181. 10.1155/2018/5037181
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47(1): 38-47. 10.1128/JCM.01206-08
- Shui IM, Kolb S, Hanson C, Sutcliffe S, Rider JR, Stanford JL (2016). Trichomonas vaginalis infection and risk of advanced prostate cancer. Prostate 76(7): 620-3. 10.1002/pros.23153
- Kushwaha B, Devi A, Maikhuri JP, Rajender S, Gupta G (2021). Inflammation driven tumor-like signaling in prostatic epithelial cells by sexually transmitted Trichomonas vaginalis. Int J Urol 28(2): 225-240. 10.1111/iju.14431
- Seo MY, Im SJ, Gu NY, Kim JH, Chung YH, Ahn MH, Ryu JS (2014). Inflammatory response of prostate epithelial cells to stimulation by Trichomonas vaginalis. Prostate 74(4): 441-9. 10.1002/pros.22766
- Han IH, Song HO, Ryu JS (2020). IL-6 produced by prostate epithelial cells stimulated with Trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages. PLoS Negl Trop Dis 14(3): e0008126. 10.1371/journal.pntd.0008126
- Han IH, Kim JH, Kim SS, Ahn MH, Ryu JS. (2016). Signalling pathways associated with IL-6 production and epithelial-mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunol 38(11): 678-687. 10.1111/pim.12357
- Han IH, Kim JH, Jang KS, Ryu JS (2019). Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells. Prostate 79(10): 1133-1146. 10.1002/pros.23826
- Chung HY, Kim JH, Han IH, Ryu JS (2020). Polarization of M2 Macrophages by Interaction between Prostate Cancer Cells Treated with Trichomonas vaginalis and Adipocytes. Korean J Parasitol 58(3): 217-227. 10.3347/kjp.2020.58.3.217
- Im SJ, Han IH, Kim JH, Gu NY, Seo MY, Chung YH, Ryu JS (2016). Inflammatory response of a prostate stromal cell line induced by Trichomonas vaginalis. Parasite Immunol 38(4): 218-27. 10.1111/pim.12308
- Kim SS, Kim JH, Han IH, Ahn MH, Ryu JS (2016). Inflammatory Responses in a Benign Prostatic Hyperplasia Epithelial Cell Line (BPH-1) Infected with Trichomonas vaginalis. Korean J Parasitol 54(2): 123-32. 10.3347/kjp.2016.54.2.123
- Kim JH, Kim SS, Han IH, Sim S, Ahn MH, Ryu JS (2016). Proliferation of Prostate Stromal Cell Induced by Benign Prostatic Hyperplasia Epithelial Cell Stimulated With Trichomonas vaginalis via Crosstalk With Mast Cell. Prostate 76(15): 1431-44. 10.1002/pros.23227
- Kim JH, Han IH, Kim YS, Noh CS, Ryu JS (2018). Proliferation of prostate epithelia induced by IL-6 from stroma reacted with Trichomonas vaginalis. Parasite Immunol 40(6): e12531. 10.1111/pim.12531
- Kim KS, Moon HS, Kim SS, Ryu JS (2021). Involvement of Macrophages in Proliferation of Prostate Cancer Cells Infected with Trichomonas vaginalis. Korean J Parasitol 59(6): 557-564. 10.3347/kjp.2021.59.6.557
- Kim SS, Kim KS, Han IH, Kim Y, Bang SS, Kim JH, Kim YS, Choi SY, Ryu JS (2021). Proliferation of Mouse Prostate Cancer Cells Inflamed by Trichomonas vaginalis. Korean J Parasitol 59(6): 547-556. 10.3347/kjp.2021.59.6.547
- Twu O, Dessí D, Vu A, Mercer F, Stevens GC, de Miguel N, Rappelli P, Cocco AR, Clubb RT, Fiori PL, Johnson PJ (2014). Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A 111(22): 8179-84. 10.1073/pnas.1321884111
- Dessì D, Margarita V, Cocco AR, Marongiu A, Fiori PL, Rappelli P (2019). Trichomonas vaginalis and Mycoplasma hominis: new tales of two old friends. Parasitology 146(9): 1150-1155. 10.1017/S0031182018002135
- Fürnkranz U, Walochnik J, Henrich B (2018). Mycoplasma hominis shows strain-dependent increase in resistance to selected antibiotics after symbiosis with Trichomonas vaginalis. J Glob Antimicrob Resist 14: 169-175. 10.1016/j.jgar.2018.04.003
- Dessì D, Delogu G, Emonte E, Catania MR, Fiori PL, Rappelli P (2005). Long-term survival and intracellular replication of Mycoplasma hominis in Trichomonas vaginalis cells: potential role of the protozoon in transmitting bacterial infection. Infect Immun 73(2): 1180-6. 10.1128/IAI.73.2.1180-1186.2005
- Xiao JC, Xie LF, Zhao L, Fang SL, Lun ZR (2008). The presence of Mycoplasma hominis in isolates of Trichomonas vaginalis impacts significantly on DNA fingerprinting results. Parasitol Res 102(4): 613-9. 10.1007/s00436-007-0796-0
- Mabaso N, Tinarwo P, Abbai N (2020). Lack of association between Mycoplasma hominis and Trichomonas vaginalis symbiosis in relation to metronidazole resistance. Parasitol Res 119(12): 4197-4204. 10.1007/s00436-020-06930-x
- da Luz Becker D, dos Santos O, Frasson AP, de Vargas Rigo G, Macedo AJ, Tasca T (2015). High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect Genet Evol 34: 181-7. 10.1016/j.meegid.2015.07.005
- Butler SE, Augostini P, Secor WE (2010). Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States Parasitol Res 107(4): 1023-7. 10.1007/s00436-010-1975-y
- Fraga J, Rodríguez N, Fernández C, Mondeja B, Sariego I, Fernández-Calienes A, Rojas L (2012). Mycoplasma hominis in Cuban Trichomonas vaginalis isolates: association with parasite genetic polymorphism. Exp Parasitol 131(3): 393-8. 10.1016/j.exppara.2012.05.003
- Fürnkranz U, Henrich B, Walochnik J (2018). Mycoplasma hominis impacts gene expression in Trichomonas vaginalis. Parasitol Res 117(3): 841-847. 10.1007/s00436-018-5761-6
- Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, Song LY, Zhong HM, Lun ZR (2006). Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol Res 100(1): 123-30. 10.1007/s00436-006-0215-y
- Margarita V, Cao LC, Bailey NP, Ngoc THT, Ngo TMC, Nu PAT, Diaz N, Dessì D, Hirt RP, Fiori PL, Rappelli P (2022). Effect of the Symbiosis with Mycoplasma hominis and Candidatus Mycoplasma girerdii on Trichomonas vaginalis Metronidazole Susceptibility. Antibiotics 11(6): 812. 10.3390/antibiotics11060812
- Rappelli P, Carta F, Delogu G, Addis MF, Dessì D, Cappuccinelli P, Fiori PL (2001). Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells. Arch Microbiol 175(1): 70-4. 10.1007/s002030000240
- Martin DH, Zozaya M, Lillis RA, Myers L, Nsuami MJ, Ferris MJ (2013). Unique vaginal microbiota that includes an unknown Mycoplasma-like organism is associated with Trichomonas vaginalis infection. J Infect Dis 207(12): 1922-31. 10.1093/infdis/jit100
- Ioannidis A, Papaioannou P, Magiorkinis E, Magana M, Ioannidou V, Tzanetou K, Burriel AR, Tsironi M, Chatzipanagiotou S (2017). Detecting the Diversity of Mycoplasma and Ureaplasma Endosymbionts Hosted by Trichomonas vaginalis Isolates. Front Microbiol 8: 1188. 10.3389/fmicb.2017.01188
- Margarita V, Bailey NP, Rappelli P, Diaz N, Dessì D, Fettweis JM, Hirt RP, Fiori PL (2022). Two Different Species of Mycoplasma Endosymbionts Can Influence Trichomonas vaginalis Pathophysiology. mBio 13(3): e0091822. 10.1128/mbio.00918-22
- Fiori PL, Diaz N, Cocco AR, Rappelli P, Dessì D (2013). Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect 89(6): 449-54. 10.1136/sextrans-2012-051006
- Margarita V, Rappelli P, Dessì D, Pintus G, Hirt RP, Fiori PL (2016). Symbiotic Association with Mycoplasma hominis Can Influence Growth Rate, ATP Production, Cytolysis and Inflammatory Response of Trichomonas vaginalis. Front Microbiol 7: 953. 10.3389/fmicb.2016.00953
- Mercer F, Diala FG, Chen YP, Molgora BM, Ng SH, Johnson PJ (2016). Leukocyte Lysis and Cytokine Induction by the Human Sexually Transmitted Parasite Trichomonas vaginalis. PloS Negl Trop Dis 10(8): e0004913. 10.1371/journal.pntd.0004913
- Vancini RG, Pereira-Neves A, Borojevic R, Benchimol M (2008). Trichomonas vaginalis harboring Mycoplasma hominis increases cytopathogenicity in vitro. Eur J Clin Microbiol Infect Dis 27(4): 259-67. 10.1007/s10096-007-0422-1
- Govender Y, Chan T, Yamamoto HS, Budnik B, Fichorova RN (2020). The Role of Small Extracellular Vesicles in Viral-Protozoan Symbiosis: Lessons From Trichomonasvirus in an Isogenic Host Parasite Model. Front Cell Infect Microbiol 10: 591172. 10.3389/fcimb.2020.591172
- Tasca T, Bonan CD, De Carli GA, Sarkis JJ, Alderete JF (2005). Heterogeneity in extracellular nucleotide hydrolysis among clinical isolates of Trichomonas vaginalis. Parasitology 131: 71-8. 10.1017/s0031182005007377
- Ferla M, Tasca T (2021). The Role of Purinergic Signaling in Trichomonas vaginalis Infection. Curr Top Med Chem 21(3): 181-192. 10.2174/1568026620999200904122212
- Menezes CB, Durgante J, de Oliveira RR, Dos Santos VH, Rodrigues LF, Garcia SC, Dos Santos O, Tasca T (2016). Trichomonas vaginalis NTPDase and ecto-5′-nucleotidase hydrolyze guanine nucleotides and increase extracellular guanosine levels under serum restriction. Mol Biochem Parasitol 207(1): 10-8. 10.1016/j.molbiopara.2016.04.003
- Primon-Barros M, Rigo GV, Frasson AP, Santos Od, Smiderle L, Almeida S, Macedo AJ, Tasca T (2015). Modulatory effect of iron chelators on adenosine deaminase activity and gene expression in Trichomonas vaginalis. Mem Inst Oswaldo Cruz 110(7): 877-83. 10.1590/0074-02760150076
- Menezes CB, Tasca T (2016). Trichomoniasis immunity and the involvement of the purinergic signaling. Biomed J 39(4): 234-243. 10.1016/j.bj.2016.06.007
- Menezes CB, Frasson AP, Meirelles LC, Tasca T (2017). Adenosine, but not guanosine, protects vaginal epithelial cells from Trichomonas vaginalis cytotoxicity. Microbes Infect 19(2): 122-131. 10.1016/j.micinf.2016.11.001
- Giordani RB, Weizenmann M, Rosemberg DB, De Carli GA, Bogo MR, Zuanazzi JA, Tasca T (2010). Trichomonas vaginalis nucleoside triphosphate diphosphohydrolase and ecto-5′-nucleotidase activities are inhibited by lycorine and candimine. Parasitol Int 59(2): 226-31. 10.1016/j.parint.2010.02.004
- Petró-Silveira B, Rigo GV, da Silva Trentin D, Macedo AJ, Sauer E, de Oliveira Alves E, Tallini LR, Garcia SC, de Souza Borges W, Zuanazzi JÂS, Tasca T (2020). Trichomonas vaginalis NTPDase inhibited by lycorine modulates the parasite-neutrophil interaction. Parasitol Res 119(8): 2587-2595. 10.1007/s00436-020-06739-8
- Ibáñez-Escribano A, Nogal-Ruiz JJ, Pérez-Serrano J, Gómez-Barrio A, Escario JA, Alderete JF (2015). Sequestration of host-CD59 as potential immune evasion strategy of Trichomonas vaginalis. Acta Trop 149: 1-7. 10.1016/j.actatropica.2015.05.003
- Paintlia MK, Kaur S, Gupta I, Ganguly NK, Mahajan RC, Malla N (2002). Specific IgA response, T-cell subtype and cytokine profile in experimental intravaginal trichomoniasis. Parasitol Res 88(4): 338-43. 10.1007/s004360100396
- Yadav M, Gupta I, Malla N (2005). Kinetics of immunoglobulin G, M, A and IgG subclass responses in experimental intravaginal trichomoniasis: prominence of IgG1 response. Parasite Immunol 27(12): 461-7. 10.1111/j.1365-3024.2005.00800.x
- Kaur S, Khurana S, Bagga R, Wanchu A, Malla N (2008). Antitrichomonas IgG, IgM, IgA, and IgG subclass responses in human intravaginal trichomoniasis. Parasitol Res 103(2): 305-12. 10.1007/s00436-008-0971-y
- Bastida-Corcuera FD, Singh BN, Gray GC, Stamper PD, Davuluri M, Schlangen K, Corbeil RR, Corbeil LB (2013). Antibodies to Trichomonas vaginalis surface glycolipid. Sex Transm Infect 89(6): 467-72. 10.1136/sextrans-2012-051013
- Ton Nu PA, Rappelli P, Dessì D, Nguyen VQ, Fiori PL (2015). Kinetics of circulating antibody response to Trichomonas vaginalis: clinical and diagnostic implications. Sex Transm Infect 91(8): 561-3. 10.1136/sextrans-2014-051839
- Yadav M, Dubey ML, Gupta I, Bhatti G, Malla N (2007). Cysteine proteinase 30 in clinical isolates of T. vaginalis from symptomatic and asymptomatic infected women. Exp Parasitol 116(4): 399-406. 10.1016/j.exppara.2007.02.007
- Hernández HM, Figueredo M, Garrido N, Sánchez L, Sarracent J (2005). Intranasal immunisation with a 62 kDa proteinase combined with cholera toxin or CpG adjuvant protects against Trichomonas vaginalis genital tract infections in mice. Int J Parasitol 35(13): 1333-7. 10.1016/j.ijpara.2005.08.010
- Hernández H, Marcet R, Figueredo M, Garrido N, Sarracent J (2008). Comparative study of epitopes recognized by two monoclonal antibodies that protects mice against Trichomonas vaginalis challenge. Exp Parasitol 118(4): 583-6. 10.1016/j.exppara.2007.10.005
- Hernández H, Sariego I, Garber G, Delgado R, López O, Sarracent J (2004). Monoclonal antibodies against a 62 kDa proteinase of Trichomonas vaginalis decrease parasite cytoadherence to epithelial cells and confer protection in mice. Parasite Immunol 26(3): 119-25. 10.1111/j.0141-9838.2004.00690.x
- Xie YT, Gao JM, Wu YP, Tang P, Hide G, Lai DH, Lun ZR (2017). Recombinant α-actinin subunit antigens of Trichomonas vaginalis as potential vaccine candidates in protecting against trichomoniasis. Parasit Vectors 10(1): 83. 10.1186/s13071-017-2009-8
- Mendoza-Oliveros T, Arana-Argáez V, Alvaréz-Sánchez LC, Lara-Riegos J, Alvaréz-Sánchez ME, Torres-Romero JC (2019). Immune Response of BALB/c Mice toward Putative Calcium Transporter Recombinant Protein of Trichomonas vaginalis. Korean J Parasitol 57(1): 33-38. 10.3347/kjp.2019.57.1.33
- Mirasol-Meléndez E, Brieba LG, Díaz-Quezada C, López-Hidalgo M, Figueroa-Angulo EE, Ávila-González L, Arroyo-Verástegui R, Benítez-Cardoza CG (2018). Characterization of multiple enolase genes from Trichomonas vaginalis. Potential novel targets for drug and vaccine design. Parasitol Int 67(4): 444-453. 10.1016/j.parint.2018.04.003
- Barajas-Mendiola MA, Castellano LE, Barrios-Rodiles M, Deveze-Alvarez MA, Avila EE, Cuéllar-Mata P (2019). Reduced Trichomonas vaginalis viability in mice pretreated with parasite DNA. Parasitology 146(13): 1636-1645. 10.1017/S0031182019001094
- Corbeil LB (1995). Use of an animal model of trichomoniasis as a basis for understanding this disease in women. Clin Infect Dis 21 (Suppl 2): S158-61. 10.1093/clinids/21.supplement_2.s158
- Nogal Ruiz JJ, Escario JA, Martinez Diaz RA, Gomez Barrio A (1997). Evaluation of a murine model of experimental trichomoniasis. Parasite 4(2): 127-32. 10.1051/parasite/1997042127
- Cobo ER, Eckmann L, Corbeil LB (2011). Murine models of vaginal trichomonad infections. Am J Trop Med Hyg 85(4): 667-73. 10.4269/ajtmh.2011.11-0123
- McGrory T, Garber GE (1992). Mouse intravaginal infection with Trichomonas vaginalis and role of Lactobacillus acidophilus in sustaining infection. Infect Immun 60(6): 2375-9. 10.1128/iai.60.6.2375-2379.1992
–
ACKNOWLEDGMENTS
T.T. thanks Conselho Nacional de Desen-volvimento Científico e Tecnológico (CNPq/Brazil) for re-searcher fellowship (T.T. 309764/2021/1).
COPYRIGHT
© 2023

Infinity war: Trichomonas vaginalis and interactions with host immune response by Bongiorni Galego and Tasca is licensed under a Creative Commons Attribution 4.0 International License.