Reviews:
Microbial Cell, Vol. 11, No. 1, pp. 187 - 197; doi: 10.15698/mic2024.05.824
From microbes to medicine: harnessing the gut microbiota to combat prostate cancer
1 Department of Anatomy, Institute of Medical Sciences (AIIMS), All India, New Delhi110029, India.
Keywords: gut microbiota, therapeutics, prostate cancer, pathogenesis, microbiome, management.
Received originally: 16/01/2024 Received in revised form: 20/03/2024
Accepted: 28/03/2024
Published: 23/05/2024
Correspondence:
Prabhakar Tiwari, Lab for Molecular Reproduction and Genetics, Department of Anatomy, AIIMS, New Delhi-11029, India; prabhakt@gmail.com
Rima Dada, Lab for Molecular Reproduction and Genetics, Department of Anatomy, AIIMS, New Delhi-110029, India; rimadadaaiims20@gmail.com
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Anjali Yadav, Meenakshi Kaushik, Prabhakar Tiwari, Rima Dada (2024). From microbes to medicine: harnessing the gut microbiota to combat prostate cancer. Microbial Cell 11: 187-197. doi: 10.15698/mic2024.05.824
Abstract
The gut microbiome (GM) has been identified as a crucial factor in the development and progression of various diseases, including cancer. In the case of prostate cancer, commensal bacteria and other microbes are found to be associated with its development. Recent studies have demonstrated that the human GM, including Bacteroides, Streptococcus, Bacteroides massiliensis, Faecalibacterium prausnitzii, Eubacterium rectale, and Mycoplasma genitalium, are involved in prostate cancer development through both direct and indirect interactions. However, the pathogenic mechanisms of these interactions are yet to be fully understood. Moreover, the microbiota influences systemic hormone levels and contributes to prostate cancer pathogenesis. Currently, it has been shown that supplementation of prebiotics or probiotics can modify the composition of GM and prevent the onset of prostate cancer. The microbiota can also affect drug metabolism and toxicity, which may improve the response to cancer treatment. The composition of the microbiome is crucial for therapeutic efficacy and a potential target for modulating treatment response. However, their clinical application is still limited. Additionally, GM-based cancer therapies face limitations due to the complexity and diversity of microbial composition, and the lack of standardized protocols for manipulating gut microbiota, such as optimal probiotic selection, treatment duration, and administration timing, hindering widespread use. Therefore, this review provides a comprehensive exploration of the GM’s involvement in prostate cancer pathogenesis. We delve into the underlying mechanisms and discuss their potential implications for both therapeutic and diagnostic approaches in managing prostate cancer. Through this analysis, we offer valuable insights into the pivotal role of the microbiome in prostate cancer and its promising application in future clinical settings.
INTRODUCTION
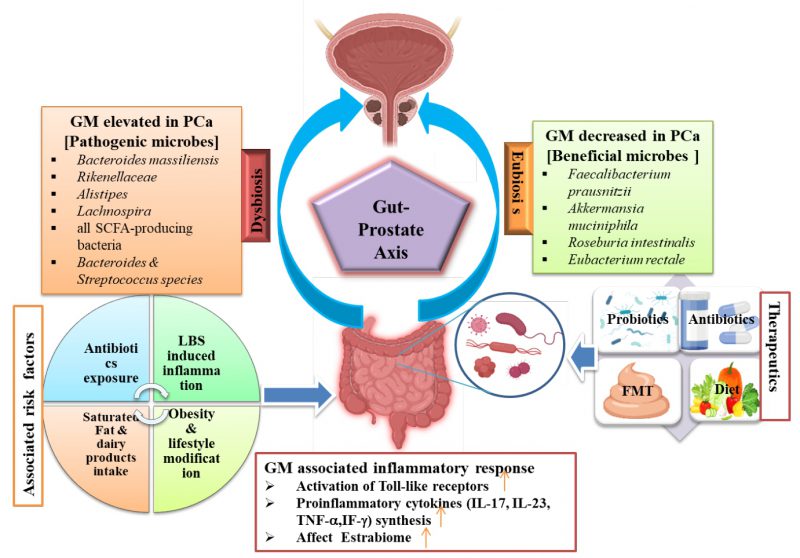
In 2020, prostate cancer (PCa) accounted for an estimated 1,414,000 new cases and 3,754,304 deaths globally, making it the second most prevalent cancer in men and the fifth leading cause of cancer-related deaths among them. Clinical detection is often delayed or undiagnosed due to its biological characteristics 1, 2. The survival rate for PCa has significantly improved in the last five years, primarily due to advancements in early detection and treatment. Currently, the human gastrointestinal (GI) microbiome is gaining attention for its potential role in cancer development and response to treatment, including PCa 3, 4. Though the gut microbiota (GM) has been linked to various diseases like rheumatoid arthritis, Alzheimer’s disease, and colon cancer, its influence on immune system modulation yields different responses to immune therapy 5. The GM, spanning various organs, is pivotal for human health. Recent advances in tools like LC-MS and genome sequencing for metabolomics and metagenomics studies have unveiled dynamic variations in microbial signatures among individuals. These variations significantly influence metabolism, host immunity, and inflammation 6. A recent study has shown that microbial pathogens can contribute to the development of approximately 15% to 20% of all types of cancers 7. The microbiota plays a crucial role in maintaining host barrier surfaces involved in local inflammation and systemic metabolic functions. An increase in GI microbiome has been linked to the induction of prostatic neoplasia and may influence estrogen metabolism 8. Certain microbiota, including Faecalibacterium prausnitzii, Bacteroides massiliensis, Bacteroides, Streptococcus, Mycoplasma genitalium, and Eubacterium rectalie have been implicated in the pathogenesis of PCa 9. Cutting-edge technologies such as next-generation sequencing (NGS) have shown the influence of the GM on PCa development and its potential implications for disease treatment 8. The development of PCa has a multifactorial etiology, with environmental and genetic factors both playing crucial roles in its progression. These factors include infection, chronic inflammation, aging, androgens, genetics, lifestyle changes and chronic diseases 10. Eating habits, obesity, and physical activity are associated with the progression of PCa. These factors are known to contribute to the dysbiosis of the body’s microflora, potentially increasing the risk of developing cancers 5. Studies have identified proteobacteria as an intestinal biomarker associated with the progression of PCa, highlighting its potential role in disease monitoring and management 11. The GM, comprising commensal bacteria and other microorganisms, constitutes the largest population of microbes in the human body 12. The relationship between humans and their GM is mutual, aiding in the development of regulatory T-cells that combat inflammation and oncogenic cells, thereby maintaining immune system balance. Sudden environmental changes can disrupt the microbiome, altering community microbial composition or bacteria abundance, potentially promoting inflammation and cancer by compromising the epithelial barrier 8. The variability of the microbiome among different patient populations has been shown to be significant in various types of cancers, including colorectal and breast cancer 13, 14. Studies have revealed that the GM is associated with PCa through both direct and indirect mechanisms 15. In a study, it has been demonstrated that modifications in the composition of the GM can increase the risk of PCa. By altering multiple cellular processes and biomarkers, including the generation of genotoxic substances and inflammatory cytokines, this can lead to disease development and modulate associated pathways 16. Despite contributing to cancer development and progression, the GM is increasingly recognized for its potential in microbiota-based diagnostics, prognostics, and therapeutics. This emerging field capitalizes on the intricate interactions between the human microbiome and cancer. Non-invasive diagnostic tools, such as faecal or salivary microbiome analysis and circulating microbial DNA, are being explored for various cancers, including PCa and testicular cancer 17. Recent advancements in GM-based therapeutics, including bacterial engineering, microbial targeting, and microbial metabolites, have emerged. For instance, in pancreatic cancer, gamma proteobacteria have been discovered to deactivate chemotherapy drugs through bacterial metabolism, highlighting the significant influence of microbial metabolites on cancer treatment efficacy 18. There are various therapeutic limitations associated with GM-related therapies, notably the complexity of the microbiome. The GM constitutes a diverse ecosystem, posing challenges in comprehending how microbes contribute to cancer development or treatment response 19. Other challenges, such as inter-individual and strain variation in the GM, contribute to the substantial heterogeneity observed in microbiome-related studies 20, 21. The current research on the gut microbiome’s involvement in PCa is hindered by the absence of standardized sampling methods and data. This impediment complicates the ability to draw firm conclusions for microbially-based cancer diagnostics, prognostics, and therapeutics. To overcome this challenge and advances in understanding of PCa, it is crucial to implement standardized sampling procedures and evaluate multiple microbiomes specimens (tissue, urine, blood, and faces) 22. Although some studies have identified specific bacteria, such as Bacteroides massiliensis, Bacteroides, and Streptococcus tissierellaceae, associated with an increased risk of PCa, the exact mechanisms by which these microbes contribute to PCa remain to be fully understood 23. Various strategies aim to transform the GM for treating PCa, including fecal microbiota transplantation (FMT), prebiotics, probiotics, or synbiotics. However, these approaches face challenges related to efficacy, safety, and patient acceptance. Therefore, identifying “favorable” or “unfavorable” microbiota is crucial for developing future microbiota therapies. Nonetheless, further research is needed to achieve this goal 24. Predicting treatment outcomes is challenging due to individual variations in GM composition, as well as genetic and dietary changes that need to be evaluated to elucidate their mechanisms 25, 26. The current understanding of potential interactions between the GM and conventional treatments is limited, as are large-scale clinical trials or cohort studies needed. This review emphasizes the significance of the GM in PCa progression and pathogenesis, including causal factors, associated mechanisms, and pathology. We also discuss GM-based therapeutic approaches and their role in diagnosing and treating PCa. We summarize the role of GM in PCa within the gut-prostate axis in Figure 1, which shows two distinct associations: eubiosis and dysbiosis.
–
–
ASSOCIATED RISK FACTORS FOR GM AND PCa
The composition of the GM varies due to both genetic and environmental factors, which can impact human health. Many studies have shown that the GM is associated with numerous non-intestinal disease 27. Although PCa is common and has well-established risk factors such as smoking, inflammation, family history, obesity, and poor nutrition, these factors may provide insights into the potential pathways involved in the development of the disease 1, 28, 29. Several studies investigating additional dietary and lifestyle risk factors have produced largely inconsistent findings. The complexity of the relationship between nutrient intake and metabolism has led to the hypothesis that discrepancies may arise from the use of imprecise surrogates for bioavailable micronutrient levels in food intake assessments 30. The composition of GI bacteria, as revealed by GM and metabolomic profiling, is influenced by environmental factors and affects nutrient availability. This GI microbiome can influence the metabolism of various substances linked to an elevated risk of PCa 1. Also, it has been associated with calcium intake from sources such as red meat, dairy products, and high-fat foods. The microbiome may play a role in the digestion of dairy products, as well as the production of phytochemicals and inflammatory molecules that could potentially impact cancer development 8. Previous research indicates that the microbiome found in different parts of the body, including the oral cavity, GI tract, and human urinary tract, may significantly influence the physiology of the prostate gland 15. In a recent study, the connection between GM and genitourinary diseases, particularly urinary tract infections and benign prostatic hyperplasia, has been demonstrated, contributing significantly to the current understanding of their association and the underlying pathophysiological mechanisms 31. The human GM, associated with numerous health conditions, presents a challenge in diagnosing Granulomatous Prostatitis (GP), as it mimics symptoms of PCa. With the increasing incidence of GP due to heightened surgical procedures and BCG utilization, meticulous attention is essential, necessitating diagnostic enhancements within the realm of urinary interventions. Furthermore, the correlation between GM and genitourinary ailments, such as benign prostatic hyperplasia and urinary tract infections, remains ambiguous, underscoring the need for additional research to elucidate the underlying pathophysiological mechanisms 32. Certain risk factors associated with PCa, such as chronic inflammation and hormonal imbalances, interact with the GM, potentially influencing disease development. Dysbiosis in the GM can exacerbate inflammation, compromise the intestinal barrier, and produce metabolites that promote cancer. These mechanisms have the potential to significantly impact the initiation and progression of PCa 27. Additionally, the GM’s influence on PCa risk and progression, through processes like chronic inflammation, microbial dysbiosis, and dietary compound metabolism, can lead to DNA damage, tumorigenesis, and immune response modulation. This intricate relationship highlights the potential for therapeutic interventions targeting the microbiota to affect PCa outcomes 33. A study involving 133 men who underwent prostate biopsy revealed higher abundance of Bacteroides and Streptococcus species in rectal swabs. Additionally, metagenomic analysis identified significant alterations in the folate and arginine pathways, suggesting a potential increase in the risk of PCa 34. In another study focusing on GM, researchers found that Bacteroides massiliensis was more abundant in men with PCa, including twelve patients at high risk and eight patients with benign prostate hypertrophy, while the levels of Faecalibacterium prausnitzii were lower 8. However, Bacteroides massiliensis is recognized for its ability to produce short-chain fatty acids (SCFA) as an anaerobic bacterium, it’s crucial to understand the intricate and interconnected nature of the GM. The decrease in Faecalibacterium prausnitzii abundance may impact overall SCFA production, given the diverse SCFA profiles contributed by various bacterial species. Bacteroides massiliensis might not fully compensate for this reduction due to variations in SCFA production patterns among bacterial taxa. Moreover, factors such as metabolic pathways, substrate preferences, and the specific types of SCFA produced (e.g., acetate, propionate, butyrate) can significantly differ among microbial species. Therefore, the lack of compensatory SCFA production by Bacteroides massiliensis could be attributed to its specific metabolic activities and its role within the complex network of the GM 1. Faecalibacterium prausnitzii has been found to metabolize acetic acid to butyric acid in the colon, which is a highly prevalent SCFA 35. Also, it primarily achieves its anti-tumor properties by inducing apoptosis and promoting cell differentiation while reducing proliferation. Additionally, in cancer cells, it inhibits histone deacetylase. 36, 37. A study on fecal microbiota showed a notable variance in the abundance of Bacteroides and Streptococcus species among individuals with and without PCa. The most noteworthy metabolic pathways observed were those associated with folic acid and arginine, crucial for nucleotide synthesis, cell growth, and DNA methylation. Insufficient folic acid levels can lead to mutations and unstable DNA, underscoring the potential impact of certain bacterial species on genomic stability in the development of PCa 34, 38. Bifidobacterium adolescentis and Lactobacillus plantarum are GM bacteria that produce folic acid, maintaining folate levels in the gut. This could impact overall health and potentially lead to cancer progression. Several studies have found that non-cancer patients harbor higher levels of folic acid-producing microflora compared to cancer patients. This suggests that folic acid from natural sources may aid in preventing PCa 12, 39. These results suggest that the GM and their metabolites play a significant role in the occurrence and progression of PCa. Modulation of GM composition has the potential to prevent the growth of lethal populations of microbes and to treat PCa. Studies have shown that different microbiota can either promote or inhibit tumor development, indicating their potential role in cancer progression and development 16, 40, 41. A well-established correlation exists between GI dysbiosis and a range of health conditions, including obesity, diabetes, and inflammatory bowel disease (IBD). Importantly, these conditions are linked to an increased susceptibility to cancer 42, 43. Specific bacterial species such as Escherichia coli, Clostridium, Bifidobacterium, and Akkermansia muciniphila have been implicated in metabolizing hormones, potentially contributing to cancer development through modulation of hormonal balance 16. A study on microbial genomics revealed significant changes in the GM composition of males with PCa compared to healthy controls 1. Modern cutting-edge genomic techniques, such as NGS and metabolomics profiling, have enabled a more extensive exploration of the metagenome, microbiota, and microbiome studies to elucidate the role of disease pathogenicity 44.
THE ASSOCIATION OF GM IN PCa PATHOGENESIS
Studies have investigated the relationship between GM and PCa through various mechanisms, including immunological regulation, metabolic alterations, and epithelial damage. Antibiotic resistance may lead to an increased survival rate of many pathogenic bacteria, including those that promote inflammation and neoplasia 8. The influence of GM on cancer development has been examined through both direct and indirect associations 15.
Direct association
Microbes directly involved in PCa
In vivo studies have demonstrated that several microorganisms may increase the risk of PCa. Similarly, the cytolethal distending toxin produced by Campylobacter jejuni has been reported to cause cell cycle arrest, cell death, and chromatin fragmentation 8. In the human intestine, E. coli often maintains a symbiotic relationship with the host. However, studies have revealed that in vivo E. coli infection can initiate a DNA damage response, highlighting potential deficiencies in DNA repair mechanisms 45. Recent research indicates that E. coli may be associated with inflammation in the prostate, potentially contributing to the development or progression of PCa 46, 47.
Effect of drugs directly on microflora
In a study, it was shown that the drugs norfloxacin, fluoroquinolones, ciprofloxacin, ofloxacin, and fleroxacin exhibited the highest capacity to penetrate prostate tissue among several medications 48, 49. Antibiotic usage can have detrimental impacts on the GM by altering metabolic processes, reducing species diversity, and changing bacterial structure. Additionally, the types of microorganisms that may respond to various quinolones can vary, leading to further variations in the GM 50. Long-term use of antibiotics can alter the structure of microbial communities, leading to interference in the activities of normal bacteria. Changes in the microbial population of the intestines or urethra have also been observed to result in alterations in the prostate microflora 12.
Indirect association
GM in phytochemical digestion
Phytochemicals are non-nutritive plant components that are physiologically active, and research has shown that they can alter the composition of gut microflora 51. Based on their metabolic origins, phytochemicals may be divided into several groups: polyphenols, alkaloids, terpenoids (both carotenoid and non-carotenoid), organosulfur compounds, and nitrogen-containing compounds 52. Phytochemicals have a positive effect on human and animal health by altering the intestinal microbiota and promoting the growth of various bacterial populations. However, changes in the composition of the GM and its metabolism of certain compounds may increase the risk of PCa. Additionally, high intake of calcium from dairy products, fat, and red meat has been linked to disease progression 53, 54. The role of the microbiome in the digestion of phytochemicals and dairy products, as well as the production of inflammatory molecules that can affect cancer development, may be linked to an increased risk of PCa and associated with the composition of the GM 51.
Estrobiomes
“Functional estrobiomes” refers to the collection of genes present in enteric bacteria that are involved in estrogen metabolism 55. β-glucuronidases and β-glucuronides play a particularly important role in the conjugation and deconjugation of estrogen. Studies have shown that estrogen levels in PCa patients are higher than those in healthy controls 8. Polycyclic aromatic hydrocarbons (PAHs) produce carcinogenic metabolites, such as radical diol epoxides and cations, when activated. Estrogen promotes cancer development and can react with these metabolites, leading to mutations that facilitate cancer growth. According to Plottel’s notion of the estrobiome, disruptions in the estrogen pathway may result in increased serum estrogen levels 55. The potential link between the risk of developing PCa and the metabolic alteration in GM has been suggested, indicating a potential positive association 8, 56. The estrobolome model suggests that certain bacteria possess the genes necessary to produce β-glucuronidase, and vice versa. Studies have shown that Eubacterium sp. lack β-glucuronidase genes, while they are abundant in Bacteroides and Faecalibacterium spp. 57. β-Glucuronidases have been associated with a higher risk of oncogenesis, as they contribute to elevated levels of xenobiotics and mutagens by deconjugating glucuronated substrates of the liver 58, 59. The absence of β-glucuronidase activity in the benign group underscores the significance of the estrobolome paradigm 55. However, further research is necessary to make conclusive remarks.
Chronic inflammation
Chronic inflammation is suggested as a potential mechanism for inducing dysbiosis, thereby raising the risk of cancer. Notably, men with a history of prostatitis are more prone to developing PCa 8. In vivo studies have confirmed that GI tract bacterial infection can increase microinvasive carcinoma and prostate intraepithelial neoplasia (PIN). The neutralization of tumor necrosis factor (TNF) prevented neoplasia onset, suggesting that inflammation based on gut microbiota contributes significantly to tumor development and progression 60. A study employing NGS to examine the rectal microbiome profiles of men before transrectal prostate biopsy discovered notable elevations in proinflammatory Streptococcus species and Bacteroides in individuals diagnosed with PCa 61. Neoplastic-related inflammation may lead to cellular and genomic damage, angiogenesis, and tissue repair on a larger scale, potentially triggering a cascade of cellular repair processes 62. It is hypothesized that during inflammation, immune cells release reactive oxygen species and reactive nitrogen species, which may directly damage cells and DNA 63. Cellular death and oxidative damage are recognized as the underlying factors contributing to proliferative inflammatory atrophy, which is regarded as a precursor to prostatic neoplasia 47, 64. The transition from a healthy microbial balance to dysbiosis plays a pivotal role in microbiota-related cancer development. The bacterial microbiome can contribute to tumorigenesis by activating Toll-like receptors (TLRs), inducing DNA damage and genomic instability via genotoxins in host cells, and modulating host gene expression through the production of metabolites with epigenetic effects 16.
The microbiota-driven inflammatory response can lead to the synthesis of pro-inflammatory cytokines such as IL-17, IL-23, interferon gamma, and TNF-alpha. This systemic inflammation can elevate the risk of inflammation at distant sites 65.
Lifestyle modification and dietary intake
Dietary habits play a significant role in PCa development. Consuming a western diet, which is characterized by high-fat dairy products, red meat, and potatoes, is associated with a higher risk of cancer. Conversely, a diet rich in high-fiber products, fruits, vegetables, and fish is linked to a lower risk of PCa 5. Additionally, being overweight increases the risk of developing the disease. Similarly, obesity is associated with various types of cancer, including PCa 29. Studies have shown that a high intake of animal protein, saturated fat, and amino acids, as well as a low intake of fiber, are positively correlated with a GM dominated by Bacteroides and Bifidobacterium. Conversely, a high intake of carbohydrates and monosaccharides is associated with a GM dominated by Prevotella 12. The GM generates a diverse array of metabolites that enter the host’s circulation, exerting various effects on the host’s overall health and well-being 66. Previous reports have indicated that the metabolism of carnitine, trimethylamine, and choline precursors by the GM raises the risk of PCa 67, 68. In summary, changes in dietary habits can exert a profound influence on the composition of the GM, impacting its diversity and balance.
THERAPEUTICS
Utilizing GM-based therapies, such as probiotics, symbiotic, FMT, and prebiotics, can modulate the gut microbiome for PCa therapy. These treatments have shown promise in transitioning PCa patients from unfavourable to favourable traits, potentially aiding in both prevention and treatment of the disease 69. Preclinical studies suggest that specific probiotics, such as Lactobacillus rhamnosus GG (LGG) and Bifidobacterium breve, can inhibit cancer cell growth, induce apoptosis, and sensitize cancer cells to chemotherapy 70. FMT), which involves transferring faecal matter from healthy donors to patients, has shown promising results in the treatment of several types of cancer, including colorectal cancer, melanoma, and GI cancers 2. As of now, clinical trials focusing on therapeutic interventions involving the GM in PCa patients are at varying stages, including ongoing trials, those in the recruitment phase, and or completed trials. These diverse stages reflect the ongoing efforts to comprehend and address the challenges specific to PCa treatment strategies targeting the GM 22. Clinical trials are currently underway to evaluate the safety and efficacy of FMT and probiotic therapy in PCa therapy. One such trial, the PROSPECT study (Probiotics to Enhance Efficacy of Chemoradiotherapy in Prostate Cancer Treatment), aims to assess the effects of probiotics on the outcomes of chemo-radiotherapy in PCa patients 71.
In a study investigating the faecal microbiota of newly diagnosed, treatment-naïve overweight and obese cancer patients (including those with breast and prostate cancer) compared to matched controls, differences were observed in beta-diversity metrics and the abundance of specific genera 72. Similarly, studies have shown that the GM influences PCa by affecting intestinal permeability. Weight loss improves permeability, which tends to slow down the progression of PCa 73, 74.
A study suggests that reducing oncologic risk in PCa may be achieved through beta-adrenergic blockade, which influences dietary composition, metabolite levels, and downstream signaling pathways 58. Another trial investigated the presence of urolithins and GM ellagic acid metabolites in the human prostate gland after consumption of walnut and pomegranate juice. The results indicated that urolithin glucuronides and dimethyl ellagic acid may be the molecules responsible for the beneficial effects of pomegranate against PCa 75. In a study, researchers investigated the impact of selenium supplementation on the composition of GM in PCa patients undergoing androgen deprivation therapy. The aim of this research is to understand how selenium affects the GM and to explore its potential therapeutic benefits in managing PCa and improving treatment outcome 76. While indicating potential microbiota variations, larger sample sizes are required to validate these findings, underscoring the necessity for further research on the influence of GI microbiome on carcinogenesis in cancer patients 72.
Antibiotics
In a mouse model of PCa, research has demonstrated that administering antibiotics inhibited the development of PCa induced by a high-fat diet 77. Antibiotics have been shown to alter the composition of the GM and reduce IGF-1 expression, both in PCa and in the bloodstream 5. IGF-1 is mostly produced by the liver and muscles and is primarily involved in cell growth and proliferation. It has been shown to play a role in PCa development, as it is released in an autocrine fashion by PCa cells and promotes their growth and survival by activating the MAPK and PI3K signaling pathways 78, 79. The activation of the MAPK and PI3K signaling pathways was reduced in a mouse model of PCa following antibiotic therapy. Rikenellaceae and Clostridiales, which generate SCFAs, were found to be decreased in the GM of mice fed a high-fat diet after antibiotic therapy 5. The overuse or misuse of antibiotics can lead to the selection of antibiotic-resistant bacteria, which may outcompete susceptible bacteria. However, it’s important to note that resistant bacteria are not necessarily more pathogenic. An overgrowth of certain bacteria linked to neoplasia and inflammation can occur, but the relationship with reduced bacterial diversity remains unclear 80. Studies have shown that infections caused by methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile can lead to increased antibiotic usage. These bacterial species are able to proliferate under conditions of microbial disruption, which can occur in various parts of the body, including the GI tract 8, 81.
Studies investigating the association between antibiotic exposure and PCa risk suggest that changes in intestinal permeability and GM induced by antibiotics may increase the risk of neoplastic changes in the prostate gland. However, the evidence for a direct link between antibiotic exposure and PCa is still unexplored and requires more research to elucidate their role in disease prevention 82, 83, 84, 85. Several studies have suggested that certain antibiotics, including penicillin’s, tetracyclines, quinolones, and sulphonamides, may be associated with a decrease in PCa risk. However, further research is needed to elucidate their role in preventing the disease and to better understand the mechanisms underlying this potential association 85, 86, 87, 88.
Probiotics
Probiotic strains have the potential to modulate the composition and metabolic activity of the GM, thereby promoting a more balanced ecosystem within the GI tract 89, 90. Currently, probiotics are under investigation as potential adjuvants in cancer treatment. Their role has been studied in various types of cancer, such as colorectal cancer, GI cancer, urinary bladder cancer, and others, aiming to elucidate their potential benefits and mechanisms of action 16, 91, 92, 93. Studies have suggested that certain bacterial species, such as naturally occurring Escherichia coli and Clostridium perfringens, can produce carcinogenic chemicals through the action of enzymes such as nitroreductase, azoreductase, and β-glucuronidase 94, 95, 96. Probiotics are a promising approach to balancing the activity of bacteria in the gut. Studies have shown that consuming fermented milk products can increase the population of beneficial Lactobacillus acidophilus in the gut of rats, resulting in lower levels of toxic enzymes and putrefactive bacteria 97, 98. LGG is often used as a supplement to conventional colorectal cancer therapy in order to promote symbiosis in the GI microbiome. Studies using animal models have shown that LGG possesses anti-inflammatory properties and can enhance tumor regression 99, 100. Several studies have demonstrated that administering probiotics after cancer treatment can reduce GI stress and replenish the microbiota, thereby potentially improving digestive health and overall well-being in cancer survivors 101. Research investigating the potential use of probiotics, such as Lactobacillus casei and its metabolite ferricrome, for PCa treatment is currently ongoing. While initial studies have indicated that ferricrome may have the ability to induce tumor cell death and activate the JNK pathway, further research is essential to fully elucidate these mechanisms and to determine the safety and effectiveness of probiotics in cancer therapy 101. Studies suggest that Lactobacilli may stimulate the immune system to eradicate cancerous or precancerous cells. However, the exact mechanism and by-products of this bacterial-mediated stimulatory effect remain unknown and require further investigation. While the potential of probiotics in cancer treatment is promising, additional research is necessary to ascertain their safety and effectiveness 102.
Faecal microbiota transplantation
FMT has been used to treat dysbiosis, IBD, and some pathogen infections, but the use of FMT is not limited to individuals with the GM trait, and its long-term safety and efficacy are still under investigation 103. FMT has been shown to preserve microbial diversity and restore the normal equilibrium of the GM, and is an effective treatment for recurrent or refractory Clostridioides difficile infection 101, 104. FMT has shown potential in decreasing colon tumorigenesis in pre-clinical studies with mice, but its effectiveness as an anti-tumor therapeutic application in humans is still being investigated 105, 106. FMT has been shown to affect the immune system, inflammation, microbial metabolites, cell signaling pathways, DNA damage, and extra-intestinal regions through blood circulation, which may have regulatory and anti-cancer effects on the human intestinal microbiota. However, further clinical trials are needed to establish its effectiveness 107. Although FMT has shown efficacy in treating various conditions, it can be challenging to manage due to the transplantation of both therapeutic bacterial species and the entire GM. Therefore, it is important to carefully monitor donors well-being and the unique composition of their GM to ensure safety and efficacy.
GM AS A DIAGNOSTIC MARKER IN PCa
Early detection and diagnosis of PCa are crucial for effective treatment and improved outcomes. However, current diagnostic tools such as digital rectal examination (DRE), biopsy, and prostate-specific antigen (PSA) testing have limitations in terms of accuracy and invasiveness. As a result, there is a growing interest in identifying new non-invasive biomarkers for PCa diagnosis 108, 109. The use of the GM potential as a diagnostic tool for PCa is based on the hypothesis that changes in the composition of the GM may lead to alterations in fecal metabolites and biomarkers, which can be detected non-invasively 110, 111. Several studies have explored the use of GM-based biomarkers for the diagnosis of PCa. For instance, a recent study identified a set of faecal metabolites (Rikenellaceae, Alistipes, and Lachnospira, which are all bacteria that produce SCFA) that demonstrated high accuracy in distinguishing between PCa patients and healthy controls 77. Studies suggest that the GM has potential as a non-invasive diagnostic tool for demonstrating high accuracy in discriminating between PCa patients and healthy controls 112.
FUTURE PERSPECTIVES
The number of studies investigating the relationship between diseases and the GM is increasing steadily, reflecting growing interest and recognition of the crucial role played by the GM in human health and disease. However, the exact role of the GM on PCa remains unclear. Recent research indicates that there may be both common and distinctive features among the GM across different disorders 113. Additionally, notable differences exist in the abundance and composition of specific GM between individuals with PCa and those without, including individuals with other types of cancer or those in good health 27. Several factors intricately shape the dynamics of intestinal bacteria, fostering diverse interactions with the host. At the center of maintaining this intricate balance within the complex interplay is the careful oversight of the host immune system and the precise regulation of intestinal microbial metabolites. However, perturbations in the composition of the intestinal microflora can disrupt this balance, potentially precipitating an immunological imbalance in the mucosa and thereby fostering conditions conducive to tumor development 23. The intrinsic heterogeneity of GM among individuals poses a substantial challenge, requiring precise identification of consistent microbial signatures linked to PCa 114. The establishment of causation over correlation demands rigorous experimental designs and robust, long-term follow-up studies to discern the genuine impact of the microbiome on PCa development and progression 115. Furthermore, the intricate interplay between GM and various factors such as genetics, lifestyle, and environmental elements introduces complexity to the research landscape. The translation of preclinical findings into effective therapeutic interventions for human subjects necessitates meticulous optimization, validation, and a comprehensive evaluation of potential risks and benefits 116. Ethical considerations loom large in the manipulation of GM for therapeutic purposes. Upholding participant well-being, ensuring informed consent, and addressing potential unforeseen consequences are paramount ethical obligations. The social and cultural implications of GM modification also warrant scrutiny 117. Mounting evidence suggests that GM and their metabolites play a crucial role in PCa, influencing critical processes such as metastasis, invasion, and tumorigenesis through multiple biological mechanisms. The regulation of GM is thought to have direct effects on the early stages of prostate epithelial cell transformation from benign to malignant, while also indirectly affecting immune surveillance 118. To advance our understanding of the aetiology and mechanisms involved in PCa disease progression, future studies should build upon current knowledge of GM’s role in the disease. Biochemical recurrence risk is a common issue following treatment for PCa. To address this challenge, there is growing interest in combining targeted therapy with microbial immunotherapy as a means of overcoming limitations associated with traditional therapies 119. Further research is needed to gain a comprehensive understanding of the precise role of the microbiome in PCa pathogenesis and prevention, in order to advance microbial tumor therapy as a potential approach for treatment, prevention, and early diagnosis of the disease. 8, 93, 120. The use of metagenomic and metabolomic analyses has provided valuable insights into the intricate composition of the disease, highlighting a wide range of microbial species and their associated metabolites 36, 121. Although the role of GM in the growth and castration resistance in PCa are recognized, however, the specific regulatory mechanisms are still unknown. Further exploration of these mechanisms could open up new avenues for identifying and managing disease mechanism. Additionally, to develop a personalized screening and treatment approach will help to evaluate the interaction between GM and various influencing factors such as lifestyle and genetics, which may increase the risk of developing the disease 5. Currently, probiotics are being investigated as a potential supplement to improve the composition of GM in PCa patients. Furthermore, prebiotics, which are non-digestible ingredients, have been shown to promote the growth of specific beneficial bacteria that improve human health 96, 102. Implementation of probiotics and/or prebiotics may reduce the risk of developing PCa and provide a promising avenue for future therapeutic options in its management.
In summary, this review highlights the important role that the GM may play in the development and progression of PCa, through its influence on chronic inflammation, immune modulation, and other pathogenic mechanisms. Emerging evidence suggests that the GM could serve as a promising target for novel therapeutic and diagnostic approaches in PCa. However, more research is needed to fully understand the complex interplay between the GM, inflammation, and PCa pathogenesis. Future studies should focus on elucidating the precise mechanisms involved and exploring the potential of GM modulation as a strategy for PCa management.
ACKNOWLEDGMENTS
Authors acknowledge the help and access to research publications pro-vided by the All-India Institute of Medical Sciences (AIIMS), New Delhi, India. We also acknowledge Mr. Tejas Wattambar, Senior Medical Writer at TCS, Pune, India, for his critical reading and review of the manuscript.
COPYRIGHT
© 2024

From microbes to medicine: harnessing the gut microbiota to combat prostate cancer by Yadav et al. is licensed under a Creative Commons Attribution 4.0 International License.