Reviews:
Microbial Cell, Vol. 11, No. 1, pp. 221 - 234; doi: 10.15698/mic2024.07.827
Characterising glycosaminoglycans in human breastmilk and their potential role in infant health
1 Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, NE2 4HH, Newcastle Upon Tyne, United Kingdom. 2 Analytical Sciences Department, Société des Produits Nestlé, Nestlé Research, Vers-Chez-Les-Blanc, Lausanne, Switzerland. 3 Department of Nutrient Technology, Société des Produits Nestlé, Nestlé Research, Vers-Chez-Les-Blanc, Lausanne, Switzerland. 4 Newcastle Neonatal Service, Royal Victoria Infirmary, Newcastle Upon Tyne, NE1 4LP, United Kingdom. 5 School of Chemistry, Faculty of Medical Sciences, The University of Manchester, Manchester Institute of Biotechnology, 131 Princess Street Manchester, M1 7DN, United Kingdom.
Keywords: breastmilk, glycosaminoglycan, chondroitin sulphate, heparin, heparan sulphate, microbiome.
Received originally: 16/01/2024 Received in revised form: 10/05/2024
Accepted: 15/05/2024
Published: 04/07/2024
Correspondence:
Melissa Greenwood, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, 3rd Floor Leech Building, Newcastle, NE2 4HH; m.greenwood3@newcastle.ac.uk
Christopher Stewart, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, 3rd Floor Leech Building, Newcastle, NE2 4HH; christopher.stewart@newcastle.ac.uk
Conflict of interest statement: CJS declares performing consultancy for Astarte Medical and receiving lecture honoraria from Nestle Nutrition Institute. He also supervises a BBSRC collaborative training partnership PhD student for which Nestlé are involved (no salary or other personal payment is provided by Nestlé). He has no share options or other conflicts. SA & PMM are employees of Socitee des Produits Nestlé S.A.
Please cite this article as: Please cite this article as: Melissa Greenwood, Patricia Murciano-Martínez, Janet Berrington, Sabine L Flitsch, Sean Austin, Christopher Stewart (2024). Characterising glycosaminoglycans in human breastmilk and their potential role in infant health. Microbial Cell 11: 221-234. doi: 10.15698/mic2024.07.827
Abstract
Human breastmilk is composed of many well researched bioactive components crucial for infant nutrition and priming of the neonatal microbiome and immune system. Understanding these components gives us crucial insight to the health and wellbeing of infants. Research surrounding glycosaminoglycans (GAGs) previously focused on those produced endogenously; however, recent efforts have shifted to understanding GAGs in human breastmilk. The structural complexity of GAGs makes detection and analysis complicated therefore, research is time consuming and limited to highly specialised teams experienced in carbohydrate analysis. In breastmilk, GAGs are present in varying quantities in four forms; chondroitin sulphate, heparin/heparan sulphate, dermatan sulphate and hyaluronic acid, and are hypothesised to behave similar to other bioactive components with suspected roles in pathogen defense and proliferation of beneficial gut bacteria. Chondroitin sulphate and heparin, being the most abundant, are expected to have the most impact on infant health. Their decreasing concentration over lactation further indicates their role and potential importance during early life.
INTRODUCTION
Human breastmilk (BM) is a complex biofluid crucial for infant nutrition and the most important factor in early infant health. BM composition is categorised across lactation into three stages: colostrum, transitional milk, and mature milk 1. Colostrum, generally high in bioactive components, has reported roles in priming of the infant’s immune system and gut microbiome, impacting both immediate and long-term health 1, 2. As BM progresses to transitional milk, bioactive components begin to decline in concentration, simultaneously there is an increase in functional nutritional factors (Figure 1) 1. The final stage, mature BM, is primarily characterised by high concentrations of nutritional compounds and low levels of bioactive components as the main purpose of BM at this stage is nutrition 1. Figure 1 demonstrates this phenomena, showing the percentage change of bioactive components from colostrum to mature milk using reported medians and averages of preexisting datasets.
–
 | FIGURE 1: Percentage change of bioactive components in breastmilk from colostrum (1-4 days pp) compared to mature breastmilk (>15 days pp) 3, 4, 5, 6. |
BM is a key source of microbial inoculation, exposing the infant up to 700 different bacterial species over the course of lactation 7, 8. The BM microbiome develops throughout lactation with colostrum notably high in diversity colonised by Weisella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus, compared to mature BM presenting increased presence of Veillonella, Leptotrichia, and Prevotella 7. Similarly, BM is composed of varying quantities of functional and bioactive components, macro and micronutrients, with 710 metabolites identified 9, 10. Despite some direct seeding of microbes, BM mostly impacts the infant microbiome by provision of prebiotic components such as human milk oligosaccharides (HMOs). Breastfed infants demonstrate a microbiome dominated by anaerobic Bifidobacterium species (Bífidobacterium breve and Bifidobacterium bifidum), promoted by HMOs. Bacteroides are also believed to consume long chain HMOs 9, 11, 12. The bioactive components in BM have been shown to exert protective effects and adapt to the infants needs over time, compensating for their deficient immune system, with recent research demonstrating the protective effects of human milk glycans against enteric infections 13, 14.
In the absence of Mothers Own Milk (MOM), BM taken from the mother and given to her infant, and Donor Human Milk (DHM), BM donated by mothers to be given to any infant, the main substitute is formula milk which is associated with health risks 15. For instance, exclusive breastfeeding (EBF) of infants demonstrated a reduced occurrence of enteric infections by approximately 50% compared to infants fed bovine milk-based infant formula 16. Furthermore, EBF preterm infants show significant reductions in morbidity and mortality compared to partially formula fed infants 16. As such, EBF during the first six months of life is recommended by the World Health Organization 17. Described as the most effective method to ensure child health, research has shown EBF during this period is positively associated with increased linear growth 17, 18. In addition, there is evidence showing EBF for three months, or longer, results in increased intelligence scores and consistent evidence showing breastfeeding of any kind provides protection against the development of upper and lower respiratory infections, obesity, childhood inflammatory bowel disease (IBD), and diabetes 19, 20. Breastfeeding also has a range of positive impacts for the mother, not limited to reducing the risk of postpartum haemorrhage and anaemia, postpartum depression, various types of cancer and cardiovascular disease 21.
The bioactive components in BM are a current topic of intense research owing to their biomarker and therapeutic potential. A particular class of interest are HMOs, indigestible complex unconjugated sugars serving to promote the growth of beneficial bacteria in the infant gut (i.e., act as a ‘prebiotic’) 22. Research suggests that the concentration of specific HMOs may be associated with protection from disease. A lack of the HMO, disialyllacto-N-tetraose, has been associated with the development of necrotising enterocolitis (NEC) in preterm infants, with protective effects of disialyllacto-N-tetraose against NEC further demonstrated using a rat model 23, 24. Similarly, 2- linked fucosylated HMOs have associations with the protection against diarrhoea in breastfed infants for their ability to inhibit binding to host cell ligands by Campylobacter jejuni, known to cause diarrhoea 25. Therefore, infants with higher levels of 2-linked fucosylated HMOs are at reduced risk of developing pathogenic specific diarrhoea 25, 26.
Glycosaminoglycans (GAGs), are complex carbohydrates present in BM throughout lactation (Figure 1) 1, 27, 28. Previous to the relatively novel research of GAGs in BM, the majority of GAG research focused on GAGs synthesised throughout the body 29. The molecular structure determines the functions of GAGs, notable roles include their critical roles in cellular processes such as cell signalling, cell hydration, regulation of cell growth and proliferation, and structural scaffolding, in addition to more pathophysiological roles, such as in pathogen infectivity, blood coagulation, wound repair, angiogenesis, axonal growth, and metastasis 27, 28, 29, 30. In recent years, there has been a noticeable increase in research on GAGs and their roles in BM. Comparable to most bioactive components, GAG content in BM declines as infants age and their needs adjust (Figure 1) 1, 31.
Upon ingestion and arrival at the small intestine, pancreatic enzymes can digest the protein core to which GAGs are bound, resulting in ‘free’ GAGs 32. These then pass relatively undigested through the gastrointestinal (GI) tract, due to a lack of endogenous host enzymes capable of degrading GAGs, and it is only upon reaching the colon and the cecum where the free GAGs can be broken down by bacterial enzymes to be used for further metabolic purposes 32, 33. GAGs are thought to be useful in pathogen defense for new-born infants, with a proposed receptor-like mechanisms for preventing the adhesion of pathogens to epithelial cells 31. Furthermore, GAG utilisation is essential for the colonisation and proliferation of gut bacteria and therefore overall health 34. Considering this, we can hypothesise GAGs may serve an important role in infant immune protection and maturation of the infant microbiome.
THE BUILDING BLOCKS OF GLYCOSAMINOGLYCANS
Within BM there are four classes of GAGs, determined by their monosaccharide composition, more specifically their repeating disaccharide units, glycosidic linkage, and the sulphation position and amount (Figure 2) 29. These are the galactosaminoglycans; chondroitin sulphate (CS) and dermatan sulphate (DS) and the glucosaminoglycans; heparan sulphate (HS), heparin (Hep) and hyaluronic acid (HA), also termed hyaluronan, which is not sulphated 27.
–
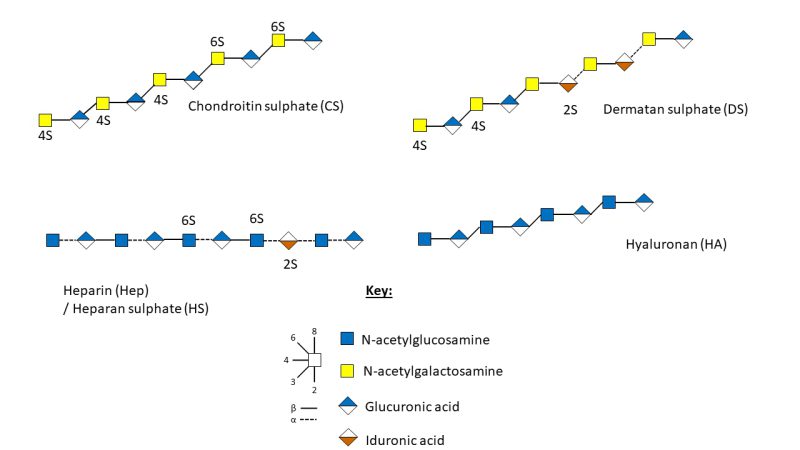 | FIGURE 2: Schematic representation of the structure and sulphation positions of chondroitin sulphate, dermatan sulphate, heparan sulphate, heparin and hyaluronic acid 35. |
As proteoglycans are transported through the secretory pathway, they can acquire multiple covalently bonded GAG chains which extend from the serine residue of ser-gly sites in the protein core through a linker tetrasaccharide (GlcA-Gal-Gal-Xyl-) 36, 37. GAG synthesis occurs de novo across the body, in a non-template driven process, and is divided between the non-sulphated biosynthesis of HA and sulphated biosynthesis of CS/DS and HS/Hep (Figure 3) 27, 30. The GAGs are composed of a uronic acid, d-glucuronic acid (GlcA) or l-iduronic acid (IdoA), and an amino sugar; N-acetyl-d-galactosamine (GalNAc) or N-acetyl-d-glucosamine (GlcNAc), with arrangement and glycosidic linkages of the monosaccharides resulting in the four classifications of GAGs, with sulphation modifications of the backbone resulting in variability 27, 38.
–
 | FIGURE 3: Simplified schematic representation of GAG synthesis in human breastmilk. UPD sugars are synthesised in the cytoplasm where they remain until biosynthesis begins. Left: non-sulphated hyaluronic acid; UPD sugars diffuse into the plasma membrane where they are polymerised by hyaluronic acid synthases, forming the HA polysaccharide. HA is then excreted unmodified into the pericellular space 30. Right: sulphated GAG synthesis of CS, DS, Hep and HS; UPD sugars are transferred to the cis Golgi by transmembrane nucleotide transporters where they act as sugar donors for glycosyltransferases 29, 30. Synthesis of the common linkage tetrasaccharide on the proteoglycan core begins in the endoplasmic reticulum (ER) and finishes in the Golgi apparatus, allowing for CS and Hep/HS chain building. CS, Hep and HS undergo sulphation and modifications in the Golgi before secretion at the trans-golgi network 30. Created with Biorender.com. |
All GAG biosynthesis begins with the biosynthesis of five uridine diphosphate (UPD) derived activated sugars within the cytoplasm: UPD-glucuronic acid, UDP-N-acetylglucosamine, UDP-galactose, UDP-N-acetylgalactosamine, and UDP-xylose 29, 30. From here onwards there is a set distinction between sulphated and non-sulphated synthesis.
HA is composed of the repeating disaccharide unit –> 4) β-D-GlcA (1–>3) β-D-GlcNAc (1–>) and may be up to 25,000 units long 30, 39. During HA synthesis UDP-glucuronic acid and UDP-N-acetylglucosamine reach the inner surface of the plasma membrane, via diffusion, where they are then polymerised by membrane-bound hyaluronan synthases 30, 40. Following synthesis, HA is secreted untethered and unmodified into the pericellular space 30 (Figure 3).
During sulphated GAG biosynthesis, UPD sugars are carried to the Golgi by transmembrane nucleotide-sugar transporters, where they act as sugar donors for glycosyltransferases 29, 30. In the endoplasmic reticulum and cis-Golgi compartment, xylosyl-transferase initiates biosynthesis of the common GAG-protein linkage tetrasaccharide: GlcAβ1-3Galβ1-3Galβ1-4Xy1-O-Ser on the peptidoglycan core protein 41. Following synthesis of the linker tetrasaccharide chain building can occur for CS and HS GAGs (Figure 3).
CS is a linear polysaccharide consisting of alternating repeating units of varyingly sulphated GalNAc and GlcA (Figure 2) 34. Initially, GalNAc is transferred to the GlcA of the linkage tetrasaccharide by GalNActT-1, followed by the alternate transfer of GlcA and GalNAc residues by GlcAT-II and GalNAcT-II, respectively, creating the CS polysaccharide backbone which can further modified by sulfotransferases 38, 41. DS formation occurs following CS modifications when GlcA C5-epimerase catalyses the epimerisation of D-GlcA to L-IdoA, resulting in conversion from CS to DS (Figure 2) 41. There are four units of sulphated disaccharides; Unit A, C, D and E. Unit A is sulphated on position O4 of the GalNAc residue in CS by four sulfotransferases; chondroitin 4-0 sulfotransferase-1, -2 and -3 (C4ST-1, -2 and -3), with dermatan 4-O sulfotransferase-1 responsible for catalysing the transfer of sulphate to GalNAc residue next to IdoA in DS 41. C and D units are sulphated by chondroitin 6-O sulfotransferase-1 on position 6 of the GalNAc residue, with unit D requiring 2-O-sulphation of the adjacent GlcA or IdoA by uronyl 2-0-sulfotransferase 41. Finally, unit E formation occurs when GalNAc-4-sulphate 6-O-sulfotransferase transfers a sulphate residue to position 6 of GalNAc(4S) 41.
Hep and HS, formed from repeating units of N-acetylglucosamine and hexuronic acid residues, are different in that 2-O-sulphated GlcA (β1-4) is linked to 6-O-sulphated-GlcNAc in HS, whereas 2-O-sulphated IdoA (α1-4) is linked to GlcNAc sulphated at N2 and O6 in Hep (Figure 2) 29, 34. Highly sulphated Hep, and its lesser sulphated counterpart HS, have the highest structural heterogeneity of all the GAGs 34, 42.
Hep and HS formation begins with GlcNAc transfer to the linkage tetrasaccharide by GlcNAc transferase-I 41, 43. Chain elongation is controlled by bifunctional exostosin (EXT) genes which encode glycosyltransferases; EXT1, EXT2, EXTL1, EXTL2 and EXTL3 44. The combined activity of EXT1 and EXT2, forming a stable Golgi-located hetero-oligomeric complex transferring GlcNAc and GlcA to the growing Hep or HS chain 41, 42, 44. Whilst EXT1 is capable of polymerising the HS backbone alone, the role for EXT2 is not fully understood, with both genes described as working together 44. There are five main pathways of biosynthetic modifications of HS and Hep chains, making them the most informationally rich GAGs 41, 42. The first two modifications; N-deacetylation and N-sulphation are essential for the subsequent reactions to take place and are catalysed by GlcNAc-N-deacetylaseIN-sulfotransferases. GlcA can then be epimerized to IdoA, with an accompanied anomeric switch (β to α) by HS C5-epimerase in the medial Golgi, the uronic acid residues can be sulphated at the O2 position by 2-O-GlcA/IdoA-sulfotransferases 41. The N-acetylglucosamine residues can be sulphated at O6 via the action of 6-O GlcN-sulfotransferases and then further sulphated at O3 via the action of 3-O-GlcN-sulfotransferases. 41. Alongside modifications of sulphation and epimerisation, de-sulphation also occurs at the cell surface and in the Golgi where endosulfatase catalyses the de-sulphation of 6-O-sulphate from 6-O sulphated HS. Chain cleavage at multiple glycosidic linkages may also take place via the action of heparinase resulting in a varied number of bioactive HS chains and chain lengths 41.
METHODS TO IDENTIFY AND CHARACTERISE GLYCOSAMINOGLYCANS
Characterising GAGs in BM remains an important research topic. Research within the GAG field is typically performed by few highly specialised research groups, with similar methodologies between studies 14, 31, 45. One reason for this is that structural complexity of GAGs makes their analysis challenging, specifically their varying sulphation patterns. Therefore, there are calls for a reliable method to be optimised for the structural characterisation and quantification of GAGs 46, 47. There are many GAG assay methods, including enzyme-linked immunosorbent assay, dye staining, mass spectrometry (MS), high-performance liquid chromatography (HPLC), and capillary electrophoresis but these require the creation of calibration curves using standards, which can be difficult to source 47, 48. Other methods include chemo- and biosensors for their rapid detection, considered satisfactory for quantification purposes, but the inability to distinguish GAG species is a limiting factor 47.
The first step to determine BM GAGs is their extraction and purification, for which some common protocols have been developed 14, 31, 45. To quantify the total GAG content in milk, the carbazole assay for uronic acids can be used. The technique dates back to 1962 and is used to determine the amount of hexuronic acids after hydrolysis of the GAGs into monosaccharides 45, 47, 49. The carbazole assay is sensitive and reproducible, allowing for the processing of multiple samples at once, with low consumption of reagents. Furthermore, it is designed for the determination of complex uronic acid-bearing polyanions such as HA, CS, DS and Hep and its derivatives 49, 50. Use of the 96-well assay allows for many samples to be processed at once, in addition to allowing for repeats to assess the coefficient of variation 50. GAGs can then be separated and quantified by agarose-gel electrophoresis combined with sequential toluidine blue (TB)/Stains-All staining, a sensitive method developed for the visualisation of GAGs 47, 51. Electrophoresis separates GAGs based on charge density and molecular size, following the use of TB for visualisation 47. Stains-All, or carbocyanine dye, stains macromolecules with specific colours and when used as a combination with TB allows for colour coded detection of GAGs 47. TB is advantageous in that it does not alter the chemical structure of GAGs and can be used to detect low levels of GAGs in tissue, but TB can react with unrelated negatively charged molecules and cannot be used for quantitative analysis of GAGs 48. Dimethyl methylene blue is another available dye and can be used both in solution or in the solid phase, however, has low sensitivity and inability to differentiate sub-species of GAGs 47, 48.
LC is a versatile common separation method used for structural characterisation of GAG disaccharides, with many applications that can be paired with multiple techniques, such as anion exchange, reversed phase anion pairing and size exclusion chromatography, one of the more common analytical techniques used for molecular weight analysis of HA 52. Notwithstanding, LC is time-consuming due to the lengthy sample preparation required prior to analysis. For successful LC analysis, GAG polymers must first be extracted from the sample then depolymerised using polysaccharide lyase enzymes: Chondroitinase ABC, Heparinase I, Heparinase II and Heparinase III, which cleave the glycosidic linkages resulting in the release of the disaccharide components 47, 53. This is a key step as purified GAGs are often too large and heterogenous, therefore they must be depolymerised prior to LC and/or MS analysis 52. The unsaturated disaccharides are then labelled/derivatized. This is most commonly achieved using fluorescent tags which label the reducing ends of disaccharides such as 2-aminoacridone, aminobenzamide, anthranilic acid and 2-aminopyridine 54. Following this labelling step, sample analysis can begin, where the efficiency fully depends on the method, with some significantly more time consuming than others. Thus, whilst LC does produce sensitive and reproducible results, in some cases, it may be better suited to small numbers of samples where possible 48.
MS is another powerful detection method for GAG analysis, which can be paired with LC and is superior in speed, accuracy, and sensitivity. Coupling MS separations are ‘essential’ for determination of expression patterns for GAG compound classes, even so there is discourse with some arguing it needs to be adapted to better suit GAGs, one disadvantage being its inability to provide sequence information for GAGs 47, 48, 52. GAGs are acidic and produce abundant negative ions. The acidic residues are most stable when ionised in the deprotonated form and as such can be analysed using negative mode MS 52. HPLC-electrospray ionisation-MS in particular is recognised for its ability to provide ‘soft ionization’ for the detection and characterisation of sulphated GAGs 55. Not all MS techniques are viable, fast atom bombardment MS, for instance causes fragmentation of sulphate groups and is less sensitive than commonly used electrospray ionisation 52. Wang et al. took a slightly different approach using LC-tandem MS with sensitive multiple reaction monitoring MS to determine trace concentrations of GAGs in human BM, again labelling the unsaturated disaccharides with 2-aminoacridone 56. LCMS, typically used for the analysis of GAG structure, is a time-consuming and labour-intensive process taking approximately one to two weeks from sample preparation to completed analysis. Benefits include its suitability for work with very small samples (pg/μL), and its high sensitivity 46. Recently developed Shotgun Ion Mobility Mass Spectrometry Sequencing (SIMMS2) is an emerging method for the sequencing of HS oligosaccharides 57. In combining IMMS, able to characterise isomeric arrangements of glycan building blocks, with a library of defined samples, SIMMS2 sequencing provides details regarding structure-function relationships of HS saccharides 57.
FACTORS AFFECTING BREASTMILK COMPOSITION
BM composition changes over the course of lactation to meet the varying demands of the developing infant. Multiple factors affect composition, not limited to the stage of lactogenesis, including the health of the mother and infant and their feedback relationship 13, 58. There are three main stages of lactation; colostrum, transitional, and mature BM, all of which have different ‘purposes’ and compositions 1, 59. Stage I lactation, secretory initiation is vastly different to later stages in that the mammary gland is capable of producing only small volumes of colostrum for around the first 72 hours 1, 59, 60. Colostrum primes the infant’s immune system through a variety of immunological components, notably, Immunoglobulin A (IgA), secretory IgA, lactoferrin, HMO’s, GAGs, leukocytes and other immunological components 1. Stage II, transitional milk, or secretory activation onset, triggered by progesterone withdrawal, is distinguished by the increase in lactose concentration and BM volume, alongside a decline in the sodium to potassium ratio 1, 60, 61. Transitional milk typically occurs around five days postpartum and can last up to two weeks. It is similar to colostrum in terms of composition, often showing a decline in bioactive components as postpartum age increases, and an increase in milk volume 1. As long as prolactin secretion and BM removal from the breast is maintained, milk secretion continues 61. Stage III, mature BM, occurring between four to six weeks postpartum, is defined by low levels of bioactive components, and heightened macronutrient and micronutrient content 1, 9. Typical composition of mature BM is approximately 87% water, 1% protein, 4% lipid and 7% carbohydrates (Figure 4) 62. Naturally, maternal factors are a key influencing factor in BM composition. For instance, BM can be influenced by the mother’s diet and maternal stores 1. Furthermore, maternal obesity, poorly managed diabetes, and stressful deliveries, may contribute to the delayed onset of lactogenesis, when considering stage II as the ‘onset’ of milk production 60, 61. In addition, socio-economics of the mother and sex of the infant may impact the composition of BM, with data suggesting underprivileged mothers will provide BM with a higher fat concentration for daughters over sons, with the reverse occurring with ‘economically sufficient’ mothers 63. In a similar study, research suggests sex may influence BM, with male infants receiving between 24-39% higher energy and lipid content compared to female infants, hypothesised to be a result of the differing growth demands between males and females 64.
–
 | FIGURE 4: The average composition of mature human breast milk of mothers who delivered term infants. Data compiled from published studies 3, 60, 69, 70. |
Another key factor impacting BM composition is gestational age (GA) of the infant. Prematurity accounts for approximately 11% of births worldwide and can affect all women, occurring both spontaneously, but also in response to health issues such as infections or chronic illness 65. Premature infants can be categorised by their GA as extremely preterm (22-28 weeks GA), ‘very’ preterm (28-32 weeks GA), ‘moderate’ prematurity (32-34 weeks GA), or as ‘late’ prematurity (35-37 weeks GA), with ‘term’ infants born beyond 37 weeks GA 65. These classifications are important to understand foetal maturity and the receipt and composition of BM. Furthermore, prior to 34 weeks GA, infants lack the ability to coordinate suckling, swallowing, and breathing and are not able to feed directly at the breast so are fed expressed milk enterally, either intermittently or continuously through the use of a nasogastric tube, where milk bypasses the oral cavity to reach the stomach 9, 66. As such, infants fed expressed milk are unable to benefit from the feedback relationship between mother and infant, an important driver for regulation of BM demand and the infant’s nutritional requirements 9. Enterally fed infants can receive fresh or frozen MOM, DHM, or formula. DHM milk is typically expressed from mothers of term infants and is highly regulated 67. Following screening, DHM is pasteurised at high temperatures, impacting the viability of some bioactive components in human BM, for instance analysis of IgA from colostrum pre- and post-pasteurisation found a decline in IgA 68. Furthermore, with MOM unable to meet the nutrient requirements of preterm infants without fortification, it is required for DHM to receive further fortification prior to being given to very low birth weight infants 69.
GLYCOSAMINGLYCANS IN BREASTMILK AND FORMULA MILK
As discussed, there are multiple variables capable of influencing the overall composition of human BM. Despite being an abundant component of human BM, few studies have quantified GAG concentration throughout lactation. Even less explored are GAG concentrations in preterm milk, likely owing to the small volume of milk samples available from such cohorts. A study by Coppa et al., quantifying GAGs in healthy mothers delivering preterm and term infants reported a gradual decline in concentration over lactation, similar to other bioactive components. Notably, BM of mothers to preterm infants had higher GAG concentrations when compared to the BM of mothers of term infants 31. For instance, at four days postpartum, BM of preterm infants contained 9.3 g/L of GAGs compared to the 3.8 g/L in BM of term infants. Similarly, at 30 days postpartum BM of preterm infants contained 4.3 g/L compared to term infants who received only 0.4 g/L 31. These results were further validated by a 2018 study, demonstrating an overall decline in GAG content as lactation progressed 56. Using BM collected from all lactation stages, analysed using LCMS, Wang et al. demonstrated seven types of HS disaccharides present in BM, accounting for 17-64% of the total GAG disaccharides, six types of CS disaccharide, accounting for 36-79% of total GAGs, and a limited presence of HA representing 1-9% of GAG content in BM 56. Whilst there was no considerable variation of HS disaccharide composition (%) over lactation, CS showed a change in the two CS disaccharide concentrations: CS-0S and CS-6S between days 30-183. Similarly, the degree of HS sulphation remained relatively consistent, with a slight increase from 1.0 to 1.5 observed around day 183. CS degree of sulphation initially increased exponentially until day 43 of lactation, then demonstrated a steady decline up to day 183 of lactation 56. A degree of intraindividual variation was also demonstrated 56.
Whilst there are many studies demonstrating the significant changeable nature of GAGs over lactation, not all data agree. A pilot study analysing 50 BM samples from mothers across East Europe, North Africa, Central Africa, South America and Asia found no significant difference in the qualitative composition of GAGs throughout lactation 71. Across the 50 samples there was a reported range of 0.018 – 1.93 g/L with a mean of 0.361 ± 0.711 g/L , closely mirroring the mature BM concentrations of GAGs reported by Coppa et al. in term infants 14, 71. Volpi et al. also reported no significant difference of GAG concentration over lactation between the various countries and ethnicities, however, with only ten samples per listed location the validity of this particular result is limited. A larger sample size for this study may have yielded results more in line with other literature. However, often the case within this field, studies with larger sample sizes are not easily facilitated, therefore more studies are needed collectively to be able to form sound conclusions.
The impact of disease can be another challenging factor to study but the use of case studies where the individual is used as their own control might help to disentangle the role of disease by overcoming patient to patient variability. Analysis of BM from both breasts of a 35-year-old mother in her second month of lactation with a papillary infiltrating carcinoma tumour in one breast demonstrated the impact of the mother’s health on GAG composition 72. CS disaccharides isolated from BM of the cancerous breast showed an increased charge density compared to those isolated from the healthy breast; 0.7 vs. 0.3 whilst, the nonsulphated / monosulphated ratio of CS isolated from the cancerous breast was significantly lower at ~0.4 compared to ~2.3 in the ‘healthy’ breast 72. Whilst there is no comparable dataset regarding the sulphation pattern of BM GAGs in ‘unhealthy’ mothers, Volpi et al. reported a mean CS charge density of 0.37, comparable to ratio of ‘heathy’ BM GAGs 71, 72. Sulphation pattern is known to encode the molecular recognition capabilities and activity of GAGs, suggesting the GAGs synthesised in the cancerous breast will have differing capabilities to those produced in the healthy breast 73. There are no studies reporting the absence of breast-to-breast variability in GAG composition of healthy mothers, so the results need to be considered with caution. Whilst there is an increasing interest for research within this field, there are also many neglected areas due to the novelty of this subject. For instance, no data was found regarding potential impacts on GAG concentrations in BM as a result of diet changes or general health.
A potential factor affecting GAGs in BM is pasteurisation, an important process ensuring the safety of DHM 67. One study investigating the impact of pasteurisation on GAGs noted no significant difference in total GAG content of non-treated and treated samples 45. They did, however, report an overall decrease of GAG content by 18% of which 17% was observed as being HS after pasteurisation. Though these results are considered non-significant, they imply there may be some negative effects on GAG content related to the pasteurisation process, or perhaps outliers due to small sample size 45. Regardless, a repeat of this study with a larger sample size would be beneficial. MOM is always the best source of nutrition for an infant, followed by DHM. In the event of an absence of MOM and DHM, formula milk provides a necessary solution. Formula milk composition varies greatly compared to human milk, where bovine milk typically as a base with many variations available, each supplemented in an attempt to better mimic the composition of BM such as prebiotics, iron, and fatty acids 74. Outcomes of EBF infants are superior to those of infants fed formula milk, as such efforts to improve formula are ongoing 75. Naturally, there are many regulations on formula milk with product reformulations requiring sufficient medical and nutritional findings 74.
In the first reported literature on GAGs in BM, Shimizu et al. demonstrated total GAG content in human milk fat globule (MFG) membranes as being five to ten times higher in BM than in formula 76. Of the GAGs present, in both formula milk and the MFG 70% was HS, with the remaining 30% formed from CS 76. The MFG originates from the mammary gland epithelia, is triglyceride rich, and enriched with bioactive components 77. Its primary ‘purpose’ being a source of nutrition providing up to 50% of the calorie content in BM, therefore, the GAGs present in the MFG are likely not fully representative of GAG composition in BM 77. Quantitative evaluation of total GAGs (as uronic acids) in BM samples showed a mean of 416.26 mg/L (± s.d. 85.6 mg/L) where approximately 55% of the overall GAG content was formed from CS with Hep forming approximately 42%, with the remaining 3% formed from DS and HA 14. In comparison, the overall GAG content in bovine milk is 60.2 mg/L, approximately seven times less than that of human BM and primarily composed of DS (40%), followed by Fast Moving Hep (29.4% of GAGs), CS (21.4% of GAGs) and Slow Moving-Hep and HA forming the remaining 9.5% of GAG, where fast moving and slow moving refer to the speed at which the GAG components run in agarose-gel electrophoresis 14, 78. These values do not correspond with those later published where CS was shown to consistently represent 60-70% of GAG content with Hep constituting the remaining 30-40% in MOM from healthy mothers who delivered term or preterm infants31. Evidence also demonstrates the GAG charge density, and distribution of sulphate groups differs between bovine derived formula and human BM 33. CS for instance is a low-sulphated polymer with a charge density of 0.44 in BM and 0.49 in faeces of BM-fed infants. However, the faeces of infants fed formula milk showed CS/DS as having a high sulphate to carboxyl ratio of 0.75, due to the high content of DS in formula milk. HS is highly sulphated, with a charge density of 1.50 in BM and 0.40 in faeces of infants fed BM, but a charge density of 0.42 in formula-fed infants 33. Furthermore, isolation and analysis of GAGs from ‘known’ amounts of BM, or formula, and infant stool collected within 24 hours of feeding implied GAGs in BM are better utilised by the infant than those in formula milk. Assuming the GAGs isolated from the milk samples represent total GAG content, in quantifying the GAGs in infant stool, this study inferred utilisation. Stool of breastfed infants contained <0.4% of GAGs, compared to formula fed infant stool which contained ~4%, therefore it was concluded breastfed infants internalise >99% of GAGs whereas formula fed infants internalise 96% of GAGs 33. Moreover, whilst HA utilisation was determined as being similar between BM and formula milk, CS, DS and HS were found to be ten to 18 times lower in infants fed formula milk 33. The overall difference in utilisation was found to be significant and whilst indicative, internalisation of GAGs (or lack of in stool samples), does not necessarily represent utilisation. To better infer this, a wider understanding of GAGs is required. Furthermore, this study operated within a 24-hour window from feeding to stool collection and whilst unlikely, it is possible not all of the ingested milk was fully digested in the time frame; therefore, the stool samples may not fully represent total GAG internalisation. Regardless, such work highlights the difference between the GAGs in BM and formula milk and the necessity of further research within the field.
Overall, GAG composition and abundance between BM and bovine-based formula milk is noticeably different. Formula fed infants receive lower quantities of utilisable GAGs, which could impact health. Thus, further research is required to understand the biological roles of GAGs in BM and their potential impact on infant health and development.
GLYCOSAMINGLYCANS: FEEDING THE GUT MICROBIOME
There are multiple factors influencing the infant gut microbiome, including mode of delivery, GA, administration of antibiotics and/or probiotics, and diet 12, 79. Indeed, the primary factor that shapes infant microbiome during the initial weeks of life is receipt of BM, due in part to the provision of ‘prebiotic’ glycans such as HMOs which are capable of regulating commensal bacteria 8, 12, 80. As such, the mother’s health can be a contributory factor to the infants developing microbiome. For instance, 16S rRNA gene sequencing of infant stool found ‘normal weight’ mothers provided infants with an enriched Firmicutes community, compared to increased Bacteroidetes in infants born to ‘obese mothers’ 81. Tangentially, this alteration of microbiota composition consequently alters the ability of GAG degradation, with infants born to obese mothers reportedly more capable of GAG degradation compared to infants born to normal weight mothers 81.
Studies exploring the complexity of the gut microbiome utilise clustered preterm gut community types (PGCTs), of which there are six 82. PGCTs 1-5 are recognised as being less diverse and dominated by Klebsiella, Enterococcus, Staphylococcus and Escherichia, whereas PGCT 6 is defined by its diversity and abundance of ‘probiotic’ Bifidobacterium and higher relative abundance of Lactobacillus 82, 83, 84, 85, 86. PGCTs 1-5 are correlated with the onset of late onset sepsis whereas, PGCT 6 (i.e., Bifidobacterium dominant) is commonly found in ‘healthy’ preterm infants, who do not go on to develop disease 82. Lactobacillus, of the order Firmicutes, are a gram-positive genus with over 200 species identified, each thought to play roles in the production of fermented products 84. Lactobacillus spp. are also shown to decrease pro-inflammatory cytokines, tumour necrosis factor-alpha, and interleukin-6, decreasing the inflammatory response, and restoring the intestinal microbiome homeostasis 8. Bifidobacterium, of the phylum Actinobacteria, are considered one of the first stable colonisers of the gut and the most enriched genera in breastfed infants, primarily due to their ability to utilise HMOs 87, 88, 89. Bifidobacterium spp. are known to modulate the inflammation and are correlated with the metabolite raffinose, (in addition to sucrose and acetic acid) which increases short chain fatty acid concentration 8, 82, 86. In order for probiotic species to have an effect they must reach their target sites whilst remaining metabolically active, with the success of colonisation relying upon their ability to utilise carbohydrates 84.
The host immune system and intestinal microbiota have a delicate symbiotic relationship responsible for maintaining intestinal health homeostasis 8. The microbiota is colonised by a vast range of species, each one with its own glycan ‘preference’, therefore the glycans consumed can directly impact the species which thrive in the microbiome, with the species able to utilise endogenous glycans exerting effects on colonic health, which can be particularly important when discussing dysbiosis 90. Dysbiosis or the disruption of homeostasis participates in antibiotic-associated colitis, obesity and risk of developing colon cancer, demonstrating the potential long-term effects of ‘improper’ gut microbiome colonisation, to which glycans have influence 90. Complex carbohydrates, including GAGs, serve as major nutrients to human microbiota but their degradation can be difficult due to their highly variable sulphation patterns 91. Hep and HS for instance are high-priority carbohydrates for Bacteroides thetaiotaomicron, major degraders for the colonic microbiota, which possess a multitude of polysaccharide lyases capable of degrading the GAGs 91. The utilisation of GAGs by genera such as lactobacilli and bifidobacteria was disputed until recent evidence demonstrated certain probiotics and bacteria as capable of degrading GAGs 84, 92. The isolation of GAG degrading bacteria from human faeces identified probiotics including Lactobacillus casei, Lactobacillus rhamnosus and Entercoccus faecalis as capable of degrading Hep, due to a genetic cluster encoding GAG metabolising enzymes 92. Some species of Bacteroides have also been identified as capable of degrading CS and HA 92. Whilst no evidence could be found proving Bifidobacterium as having GAG degrading properties, interestingly, data show the Bifidobacterial genome content as reflecting glycan availability 89.
GLYCOSAMINOGLYCANS: DISEASE AND INFECTION MANAGEMENT
The glycome, total glycans synthesised by a cell, tissue, or organism, is hypothesised to contribute to disease prevention and treatment 11, 40. Notable glycans within this group present in BM include glycoproteins; N-linked and O-linked glycans, HMOs and GAGs 11, 40. It is widely accepted that these glycans can have many beneficial functions including the inhibition of pathogen binding, attenuation of inflammation, and the growth of beneficial bacteria of the microbiota, which in turn allows for additional immune related benefits 93. However, only a few studies on GAGs present in milk are available and an important research direction will be to determine the roles of BM-derived GAGs in preterm and term BM 14, 31, 33, 45, 56, 71, 72, 76. With our understanding continuing to evolve, naturally many of the proposed functions of BM-derived GAGs come from GAGs produced across the body and other glycans. Recent research demonstrates BM-derived GAGs as serving anti-viral roles likely due to their sulphation patterns and carboxyl groups, allowing them to interact readily with biological components 94, 95. Sulphation of GAGs is an essential posttranslational modification that determines their biological properties and functions 96. Endogenously produced sulphated GAGs, specifically CS, are a major component of the extracellular matrix (ECM) of cells where their highly organised sulphation codes allow them to interact with specific growth factors, cytokines and chemokines to trigger pathways for cell growth, differentiation, anti-inflammatory responses and anticoagulation 96. One example of this, presented by Newburg et al. is the unique ability of CS, isolated from BM, to inhibit the binding of the HIV envelope glycoprotein GP120 to the cellular CD4 receptor, the essential first step in HIV infectivity 97. However, the success rate of this is variable with a reported 8-44% inhibitory effect in HIV-1 infection implying differently sulphated GAGs may have more impactful roles 97, 98. One such example of this is the role of CS isomer CS-E in angiogenesis 99. CS-E is sulphated at the C-4 and C-6 positions of N-acetylgalactosamine which allows it to selectively interact with the numerous growth factors, responsible for angiogenesis regulation 99. Another example of the sulphation code determining roles of GAGs, demonstrated via mouse and cell line studies, is the ability of the cell adhesion molecule contactin-1 to selectively bind CS-E but not CS-A, CS-C or HS for the promotion of neurite outgrowth towards primary neurons 100. Similarly, CS-A and CS-E, specifically behave as cell surface receptors for pathogens such as Plasmodium falciparum 96. Other examples of GAGs impairing cell binding expand to the human cytomegalovirus, respiratory syncytial virus, and human rotavirus 94, 101. In a similar mechanism, Hep is able to inhibit the attachment of Neisseria meningitides to host cells, demonstrated by the ‘satisfactory outcomes’ of meningococcal septicaemia patients receiving Hep 102, 103. Additionally, Hep has established anticoagulant properties, which paired with their anti-inflammatory effects are thought to aid its effectiveness in treatment of vascular diseases 104. Hep found in mast cells was also demonstrated as an important allergic mediator, triggering neuropeptides and cytokines 105. Furthermore, intravenously administered Hep has been shown to have beneficial effects on the treatment of ulcerative colitis, with similar positive results reported when HS-derived compounds were used as anti-inflammatory agents 106, 107. Notably, mammalian sulphated HS has been shown to regulate leukocyte rolling along the surface of inflamed sites, through its ability to bind leukocyte L-selectin 108. However, endogenously produced GAGs can also be detrimental, serving as prime targets for pathogens for promotion of attachment and invasion of host cells 11, 103. GAG subversion is a pathogenic strategy utilised by viruses, bacteria, parasites, and fungi to promote their attachment and invasion of host cells, move between cells, and provide protection against the host immune response 103. Similarly, HA is able to interact with cell surface receptors including CD44 expressed on the surface of most stem cells, including cancer stem cells and RHAMM, a major HA-binding protein, where it promotes locomotion, proliferation, signalling and the progression of cancer 40, 109. Unfortunately, high HA levels in cancer patients are associated with poor prognosis, further demonstrating the possible negative effects of GAGs 40. CD44 is known to mediate leukocyte rolling, with changes in expression often contributing to tumour growth, as demonstrated by an increase of HA around sites of tumour progression, as well as sites of inflammation as a result of chemokines and growth factors which simultaneously regulate HA synthesising enzymes and degrading enzymes 27, 109.
The intestinal barrier is key to maintaining health; disturbance of this barrier can result in the onset of a number of diseases 110. Studies suggest GAGs may act as soluble receptors competing with pathogens for adhesion to the intestinal cell wall, in doing this GAGs would serve as defensive factors against infections 95. Gastroenteritis, a lethal condition accounting for approximately 10% of paediatric deaths, has multiple etiological agents of disease including Staphylococcus aureus, Salmonella, Shigella, rotavirus and E. coli but in order to promote infection they must first bind to host cell monolayers 95, 101. In an in vitro study using Caco-2 and Int-407 cell lines, Coppa et al. demonstrated the anti-adhesive properties of GAG. Cells incubated with 1.5 mg/mL of HM GAGs, levels within the expected range found in term BM, showed a significant reduction in percentage adhesion of Salmonella fyris, to both Caco-2 cells Int-407 cells, and E. coli to 0119 Int-407 cells 3, 95. This inhibitory action against S. fryis etc. reduces the likelihood that infants may develop gastroenteritis 94, 95. There is also evidence suggesting human milk GAGs may contribute to the altered binding capabilities of the anti-Zika Virus and Usutu virus properties influenced by colostrum 111. In most cases, adhesion is the first step to infection, therefore, this GAG interaction may be key to certain aspects of infant health 95.
NEC, another GI disease, is the leading cause of death in preterm infants, with onset heavily associated with enteral feeding, prematurity, hypoxic-ischaemic injury and abnormal bacterial colonisation 112. The exact mechanism behind onset of NEC is unknown, however, it is believed to be initiated by stimulation of toll-like receptor 4 by gram-negative bacteria in the ileum, consequently leading to inflammation, and the subsequent release of proinflammatory cytokines and chemokines resulting in impairment of the intestinal barrier 16. By consequence, this allows for more bacterial antigens to interact with the mucosal immune system, resulting in neutrophil recruitment, a byproduct of which is the production of reactive oxygen species 16. Research demonstrates their production and subsequent release of endotoxins, not only promotes further inflammation eventually leading to a positive feedback loop, but it also results in a loss of CS and disrupts synthesis of sulphated GAGs, highlighting their potential role in NEC 110. Whilst there is no single effective treatment, it is well reported that early and EBF results in lower incidences of NEC in premature infants, likely owing to the high content of bioactive components, of which GAGs are relevant for their protective roles in intestinal inflammation, bacterial adhesion and their promotion of a healthy intestinal barrier 110, 113, 114, 115. Gut dysbiosis cannot be attributed to a single bacterium, however, analysis of infant NEC stool samples shows a majority of E. coli, for which GAGs are shown to have anti-adhesive properties, and Salmonella enterolitica 95, 115. In particular, CS is thought to have potential applications as a preventative therapy for its unique ability to increase sulphate-reducing bacteria with research demonstrating supplementation with CS increases beneficial bacteria such as Bacteroides, and reduces harmful variants in the gut, namely Staphylococcus 96, 113, 115.
Another GI disease where onset is associated with the remodelling of the intestinal ECM, is IBD 116. In a pre-print article by Francis et al., intestinal CS isomer sulphation signatures in IBD paediatric and young adult patients was characterised, using a combined LCMS approach 116. In patients suffering from IBD, monosulphated CS-A and disulphated CS-B (DS) were found to be significantly decreased. However, monosulphated CS-C, and disulphated CS-E, both implicated in inflammation and accelerated turnover, were shown to be significantly increased 116. Alternatively, in healthy control patients an abundance of the stabilising isomer CS-A, was found to be in abundance. Moreover, in healthy patients there were no patterns of hypersulphation 116. Together, these results allude to the importance of sulphation patterns in GAGs and their subsequent roles in disease progression. It is unlikely that CS isomers are the only GAGs relevant to GI disease progression, therefore, more research within this area is required to have a better understanding of the implications of GAG composition in human BM.
SUMMARY AND OUTLOOK
GAGs in human BM have a reported presence at all stages of lactation. Similar to other bioactive components, the quantity of GAGs in BM is negatively associated with postpartum age of the infant, with one study reporting increased concentrations of GAGs as a result of prematurity 31, 56. These GAGs have many suspected roles, including providing newborn infants with a pathogen defense mechanism as a result of their ability to interact with microbial pathogens via receptor-like-mechanisms 117. Moreover, GAGs may act as important carbon sources for the colonisation and proliferation of beneficial gut bacteria (i.e., probiotic), which may in turn contribute to the wider health of the infant 34. Much of the research discussed within this review comes from human studies, however, due to an overall lack of research on human BM GAGs, some results come from animal studies and implied roles from endogenously produced GAGs.
Current detection methods for GAGs in BM require time and volume consuming sample preparation and show low throughput for analysis. Further development of rapid methods requiring small sample volumes is required to enable efficient, streamlined research. A better understanding of how GAGs interact with the host organism and the gut microbiome may help us maximise infant health.
ACKNOWLEDGMENTS
MG is funded by a BBSRC collaborative training partnership PhD studentship. CJS is supported by the Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 221745/Z/20/Z), a Newcastle University Academic Track (NUAcT) Fellowship, and the 2021 Lister Institute Prize Fellow Award.
COPYRIGHT
© 2024

Characterising glycosaminoglycans in human breastmilk and their potential role in infant health by Greenwood et al. is licensed under a Creative Commons Attribution 4.0 International License.









