Back to article: Aminoglycoside resistance profile and structural architecture of the aminoglycoside acetyltransferase AAC(6’)-Im
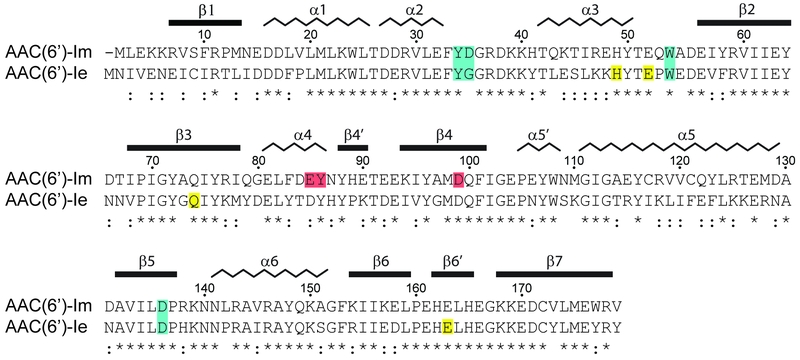
FIGURE 1: Sequence alignment of AAC(6’)-Im and AAC(6’)-Ie. The secondary structure assignment for AAC(6’)-Im is shown above, and the identity (*) and similarity (:) is indicated below each block of sequence. Amino acids involved in kanamycin A binding are shaded blue for those which are common to both enzymes, red for amino acids which interact with kanamycin A only in AAC(6’)-Im, and yellow for amino acids which interact with kanamycin A only in AAC(6’)-Ie [12]. The AAC(6’)-Im enzyme is one residue shorter than AAC(6’)-Ie, so for the sake of simplicity, the AAC(6’)-Im sequence numbering begins at Met2 so that the sequence numbering is the same for both enzymes.
[12] Smith CA, Toth M, Weiss TM, Frase H, Vakulenko SB (2014). Structure of the bifunctional aminoglycoside-resistance enzyme AAC(6′)-Ie-APH(2″)-Ia revealed by crystallographic and small-angle X-ray scattering analysis. Acta Crystallogr D70(10): 2754–2764. https://doi.org/10.1107/S1399004714017635