Back to article: A novel mechanism involved in the coupling of mitochondrial biogenesis to oxidative phosphorylation
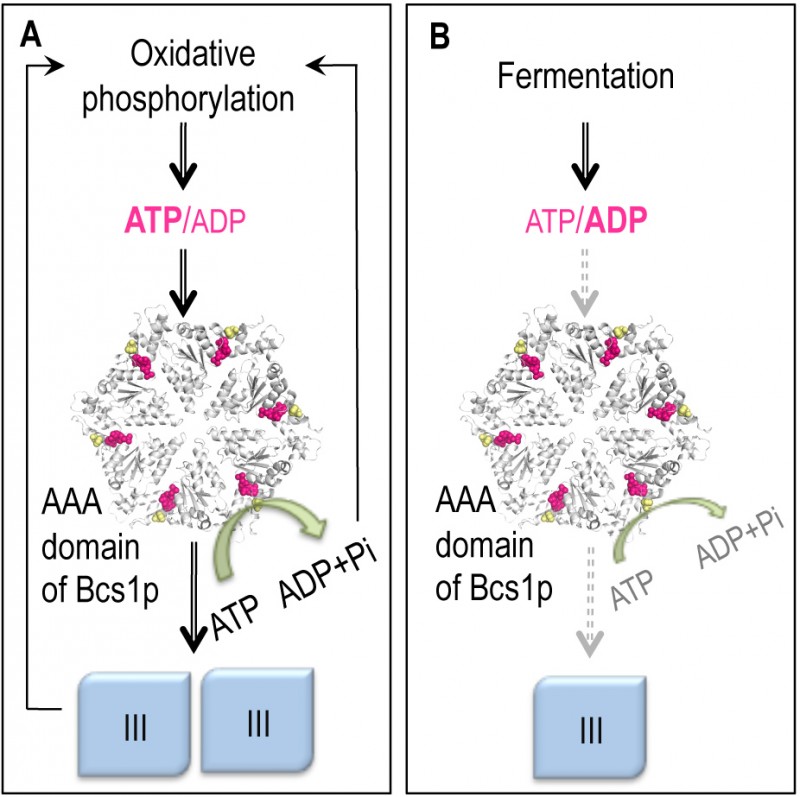
Model of the coupling of complex III biogenesis to the intra-mitochondrial pool of adenine nucleotides. The complex III is shown in blue. The predicted structural model of the putative hexamer of the AAA domain of the yeast Bcs1p is shown in white. The positions of the residue F401 (yellow) and the nucleotide binding sites (pink) are indicated. The bcs1-F401I mutation is the equivalent of the bcs1l-F368I found in a human patient and its respiratory defect is compensated for by mutations in the catalytic subunits of ATP synthase. The figure was generated with the PyMOL v1.3 software.
(A) During oxidative phosphorylation substantial amounts of OXPHOS complexes are needed; the mitochondrial ATP/ADP ratio is high, which allows rapid ATP hydrolysis by Bcs1p and the consequent assembly of complex III.
(B) During fermentation, ATP is produced by substrate level phosphorylation and there is no need to produce large amounts of the OXPHOS complexes. Under these conditions the mitochondrial ATP/ADP ratio and the ATP hydrolysis by Bcs1p are low, complex III formation is downregulated.