Back to article: The last two transmembrane helices in the APC-type FurE transporter act as an intramolecular chaperone essential for concentrative ER-exit
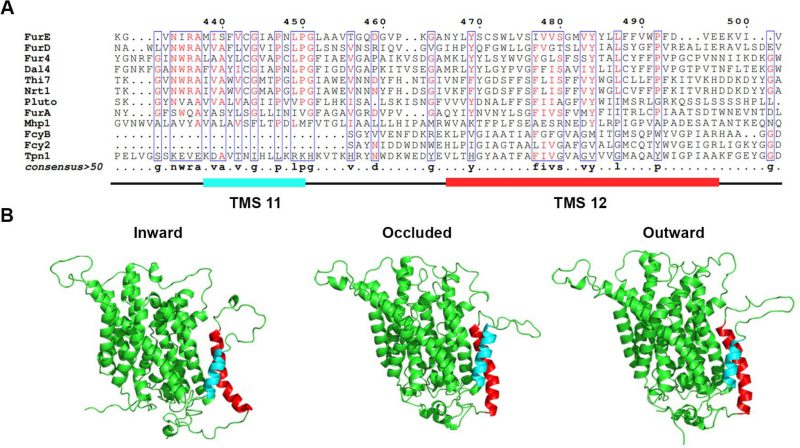
FIGURE 1: TMS11-TMS12 form a distinct structural entity topologically distinct from the LeuT-fold. (A) Structural models of FurE in three conformations have been produced through homology modelling using the crystallographically resolved structures of its bacterial homolog Mhp1 as a template. TMS11 and TMS12 are highlighted in cyan and red, respectively. PyMOL 2.5 was used for structure depiction. For details on model construction see methods. (B) Multiple sequence alignments of a segment of representative NCS1 family transporters from fungi, plants and bacteria, including TMS11 and TMS12. Positions with >50% identity are presented in boxes. For uniport IDs and taxonomy see Supplementary Table S7.