Back to article: The last two transmembrane helices in the APC-type FurE transporter act as an intramolecular chaperone essential for concentrative ER-exit
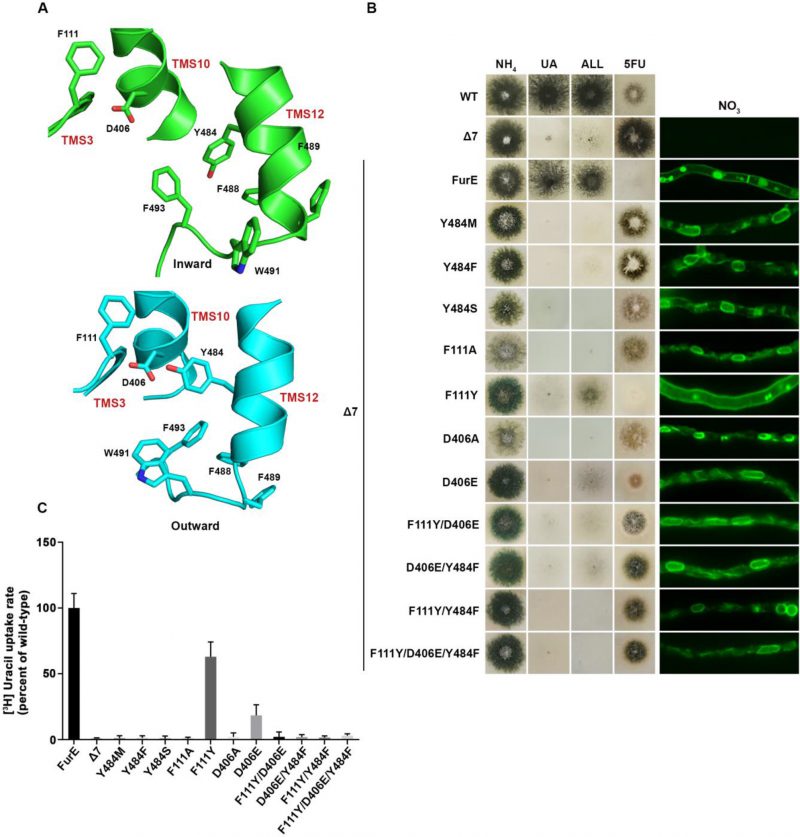
FIGURE 3: Y484 is irreplaceable for proper folding and ER-exit, mediating critical interactions between the LeuT-fold and TMS12. (A) Structural models of FurE in the inward- and outward-facing conformations highlighting important interactions in the Tyr484 neighbourhood. Notice the tilt of TMS12 between the two conformations that directs Tyr484 closer to Asp406 (TMS10) and Phe111 (TMS3) in the outward conformation. PyMOL 2.5 was used for structure depiction (see also Supplementary figure S2). (B) Growth tests and epifluorescence microscopy of control strains and strains expressing GFP-tagged FurE mutations in Tyr484 (TMS12), Phe111 (TMS3) and Asp406 (TMS10). Growth tests were performed as described in figure 2A. Scale bar for microscope images is 5 μM. (C) Radiolabeled uracil uptake rates, expressed as % of wt FurE rate, of strains expressing FurE mutant versions. For details see Materials and Methods.