Back to article: The last two transmembrane helices in the APC-type FurE transporter act as an intramolecular chaperone essential for concentrative ER-exit
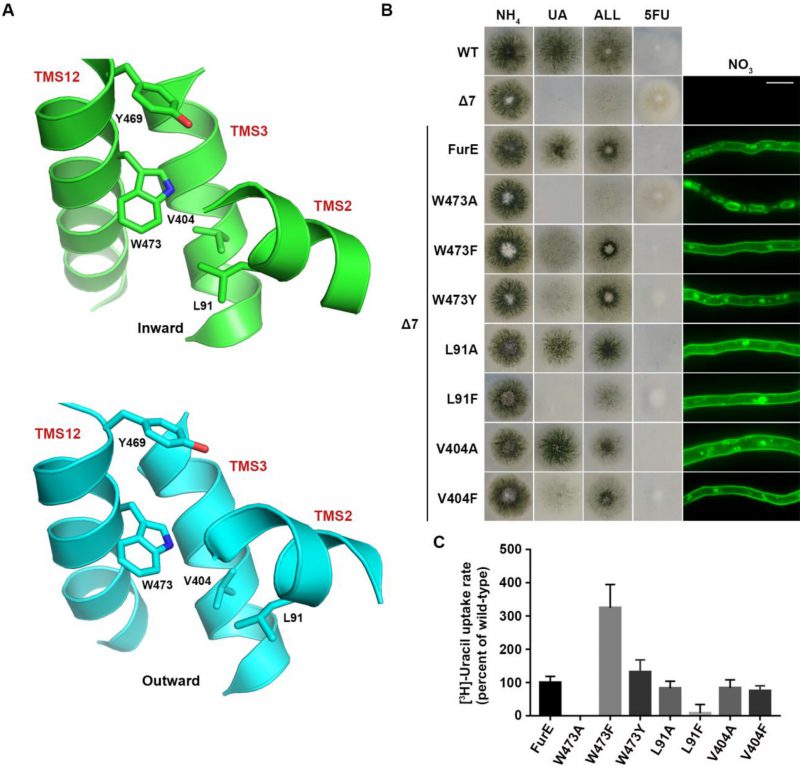
FIGURE 4: The aromatic character of W473 is critical for FurE folding. (A) Structural models of FurE in the inward- and outward-facing conformations depicting putative hydrophobic interactions of Trp473 (TMS12) with Val404, (TMS10), Leu91 (TMS2) and Tyr469 (TMS12). PyMOL 2.5 was used for structure presentation. (B) Growth tests and epifluorescence microscopy of controls and strains expressing GFP-tagged FurE mutations in Trp473, Leu91 and Val404. Scale bar for microscopy is 5 μM. (C) Radiolabeled uracil uptake rates, expressed as % of wt FurE rate, of strains expressing FurE mutant versions. For details see Materials and Methods.