Back to article: The cAMP-PKA signalling crosstalks with CWI and HOG-MAPK pathways in yeast cell response to osmotic and thermal stress
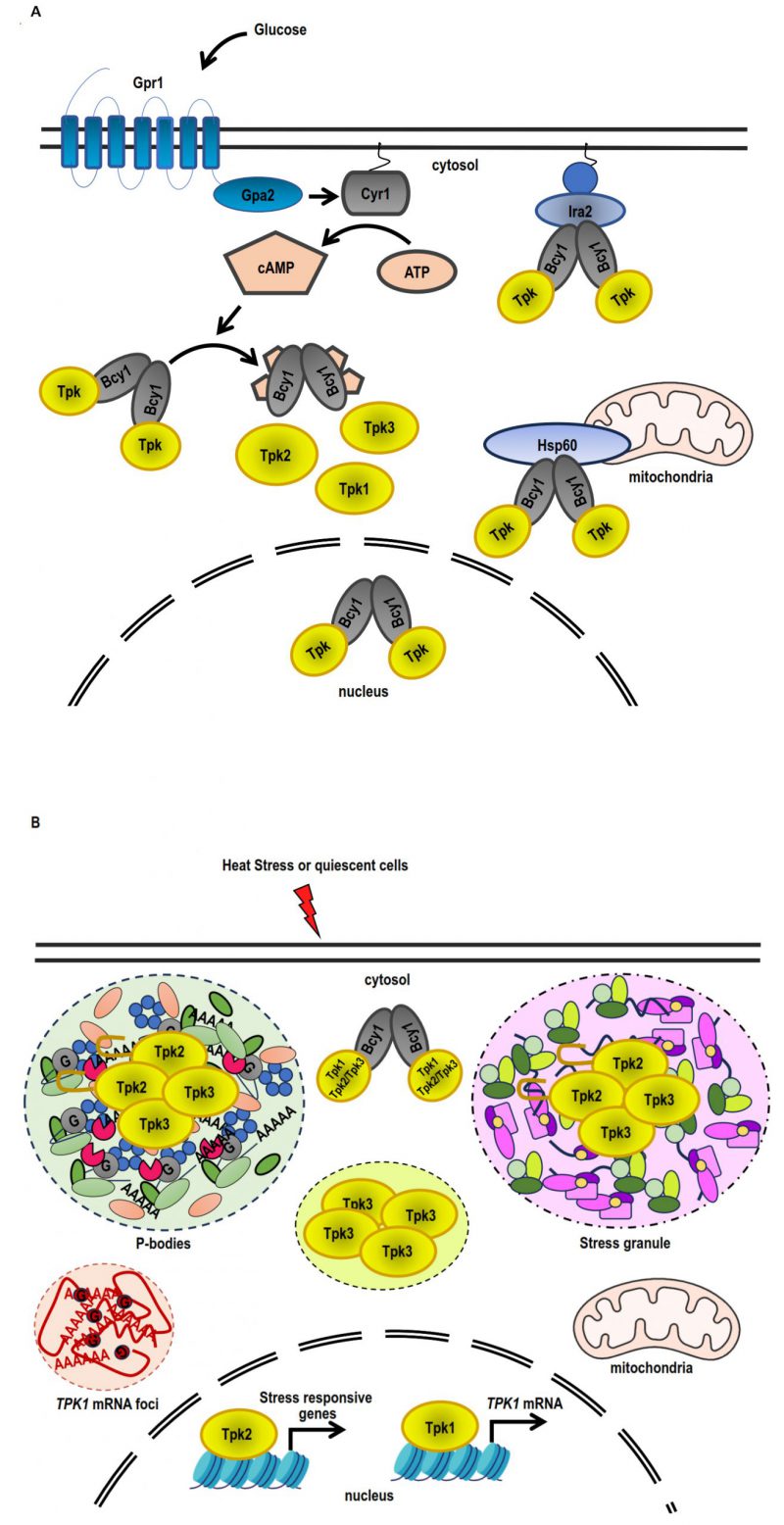
FIGURE 3: Differential PKA subunit regulation in response to stress and quiescence. (A) Under conditions of exponential growth on glucose, Bcy1 and Tpk2 are mostly localised within the nucleus, while Tpk1 and Tpk3 are found in both the nuclear and cytoplasmic compartments. The PKA holoenzyme (Bcy12-Tpks2) is tethered to the membrane-attached Ras complex by Ira2, whereas the association of the holoenzyme to the mitochondria is facilitated by Hsp60. Adding glucose causes the Ras complex and the heterotrimeric G protein-coupled Gpr1 receptor to stimulate adenylate cyclase (Cyr1), which increases the amount of cAMP inside the cell. An increase in cAMP levels triggers the dissociation of the PKA holoenzyme into a dimer of Bcy1 and two Tpk catalytic subunits, which are able to phosphorylate substrates. (B) Tpk2 and Tpk3 localise with P-bodies and/or stress granules in the cytoplasmic foci during quiescence or heat stress. The prion-like domain in N-terminus of Tpk2 facilitates its localisation into P-bodies. Tpk3 localises to foci in the cytoplasm that are distinct from conventional SGs or PBs. The locations of Tpk1 and Bcy1 in the cytoplasm are diffuse. Tpk1 and Tpk2 interact with stress-responsive genes, such as the TPK1 promoter, inside the nucleus. Heat stress triggers an increase in TPK1 mRNA expression, which subsequently accumulates in cytoplasmic foci. Foci sequestration of TPK1 mRNA inhibits translation entry.