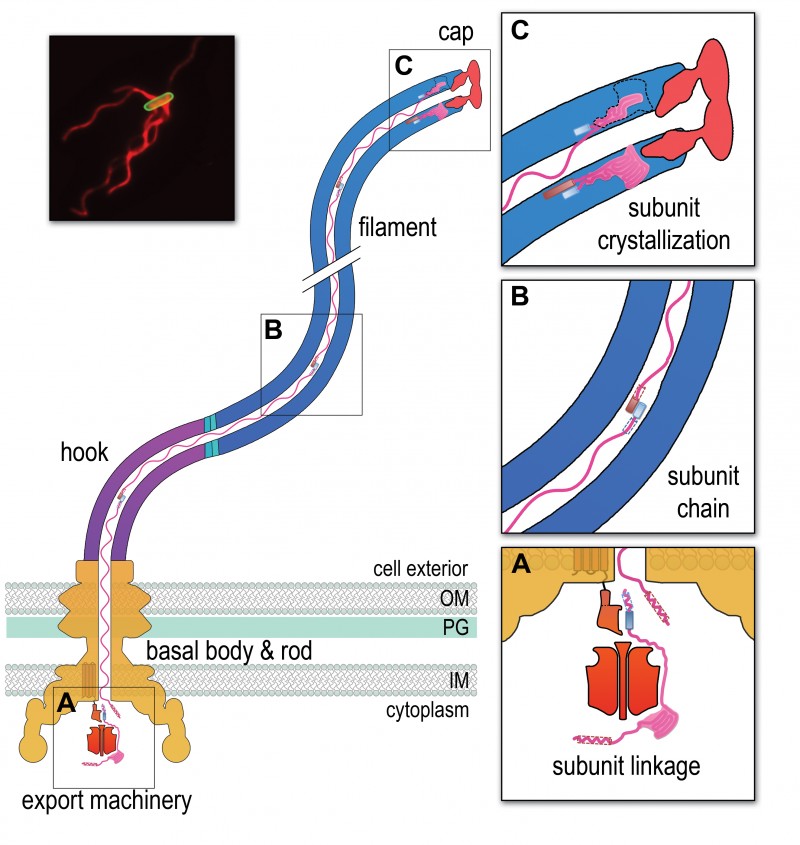
FIGURE 1: A chain mechanism for bacterial flagellum growth. Flagella on the surface of the bacterial cell (inset top right, Salmonella cell membrane stained green and flagella stained red, visualized by epifluorescence microscopy) comprise three contiguous substructures – the basal body, hook and filament – that are assembled sequentially. A central channel runs through these substructures to the flagellum tip. The basal body houses the flagellar export machinery (A) and the rod (FliE, FlgB, FlgC, FlgF and FlgG), which extends from the inner membrane (IM) and crosses the periplasm, peptidoglycan (PG) cell wall and outer membrane (OM). The cell surface hook (FlgE) is a flexible universal joint that connects the external flagellum filament (flagellin, FliC) to the basal body.
(A) Subunits of the rod, hook and filament link head-to-tail at the cytoplasmic membrane export machinery. Subunits are first unfolded by the export ATPase complex (red) and then dock at the FlhB export gate (orange). The N-terminal helix of the docked subunit is then captured by the free C-terminal helix of an exiting subunit in the flagellum channel.
(B) Sequential subunits are linked head-to-tail in a chain by juxtaposed terminal helices forming parallel coiled-coils. The resulting chain of unfolded subunits is connected through the flagellum central channel to the distal tip of the flagellum.
(C) The subunits in the chain transit from the gate to the tip. Subunits fold and incorporate into the flagellum tip beneath cap foldases (FlgJ for rod, FlgD for hook and FliD for filament subunit assembly). Subunit folding and/or crystallization not only provides a strong anchor at the flagellum tip, it also shortens the chain in the channel thus exerting a pulling force on the next subunit at the export gate, pulling it from the gate. The pulling force then drops rapidly as the new unfolded subunit enters the channel. This process repeats for each subunit captured into the chain.