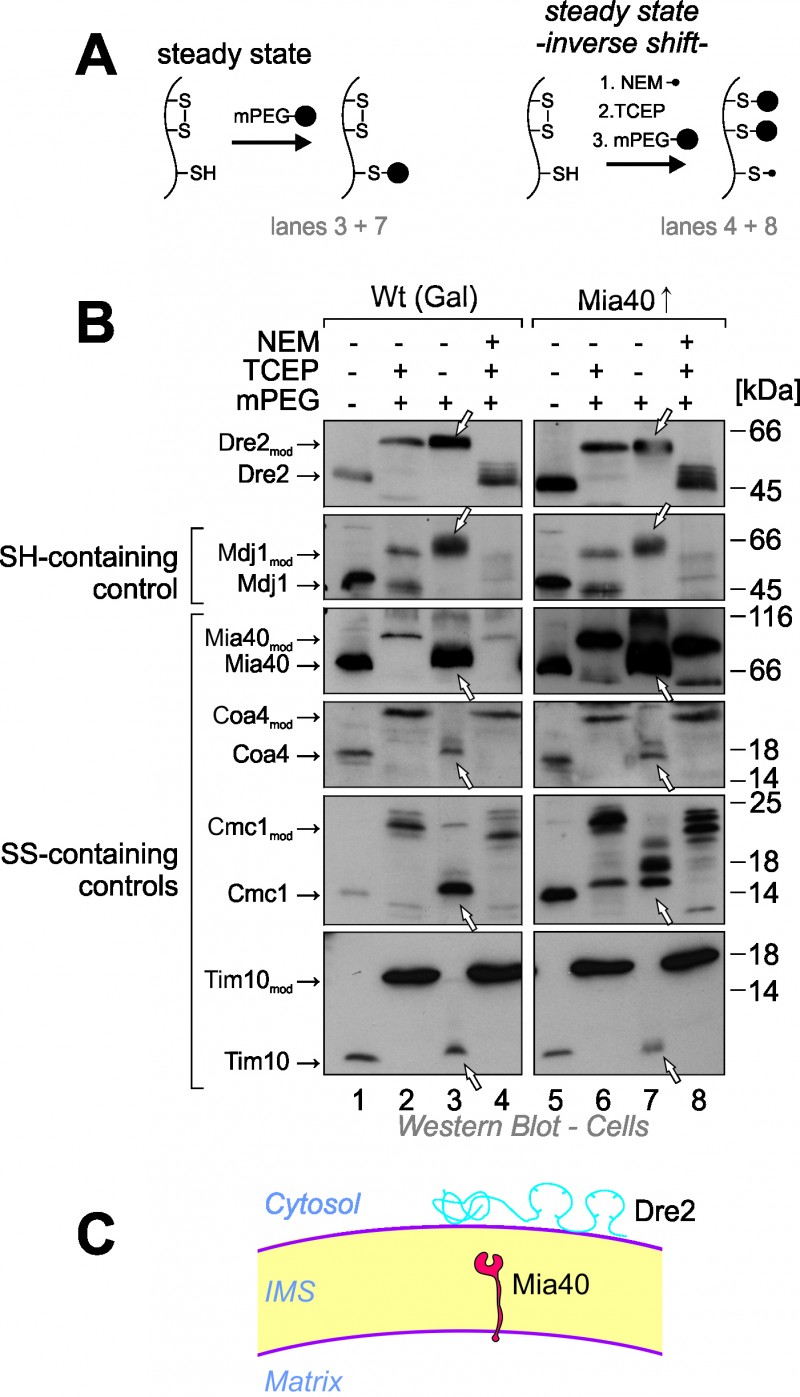
FIGURE 3: The cysteine residues in Dre2 are reduced at steady state conditions.
(A) Scheme showing the modifications of reduced and oxidized protein thiols by treatment with the alkylating agents mPEG and NEM and the reductant TCEP.
(B) Wild type or Mia40-upregulated cells in which Dre2 was overexpressed were harvested, acid treated with TCA to preserve the redox state of thiol groups and incubated with the indicated chemicals in subsequent reactions. Lanes 1 and 5 show non-modified proteins thereby serving as size standards for completely oxidized proteins. Lanes 2 and 6 show complete alkylation with mPEG-24 and hence the size expected for proteins in which all cysteines are reduced. Lanes 3 and 7 show the steady state of the proteins in the cell, lanes 4 and 8 the inverse shift after blocking thiols with NEM and opening of disulfides with TCEP. Arrowheads point at the signals of the steady state species which indicate that all thiols in Dre2 are present in the reduced form.
(C) Schematic representation of Dre2 on the mitochondrial surface. Reduced cysteine residues are indicated. Our observations suggest that Dre2 does not contain structural disulfide bonds.