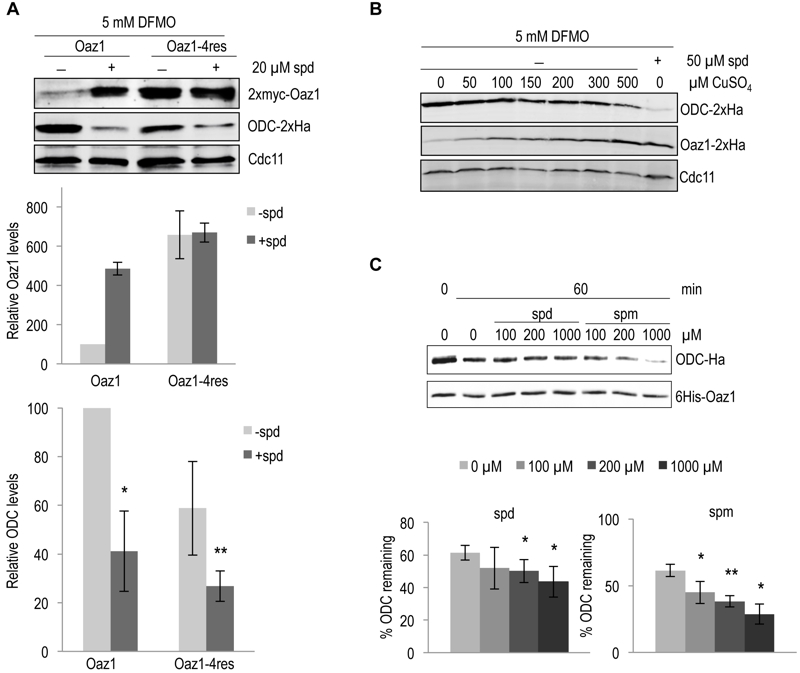
FIGURE 3: Degradation of ODC by the proteasome is directly enhanced upon polyamine addition.
(A) S. cerevisiae spe1-∆ oaz1-∆ cells were transformed with plasmids encoding ODC-2xHa and either wild-type 2xMyc-Oaz1(wt) or its 4res mutant variant. The latter proteins were encoded by in frame versions of the gene (OAZ1-if) that did not require ribosomal frameshifting during decoding. Western blot analysis of the steady state levels of ODC (middle panel) in the presence of either Oaz1 or Oaz1-4res (top panel) from yeast cells grown in the presence of 5 mM DFMO. 20 µM spermidine (spd) was added as indicated. Also shown is the quantification of the 2xmyc-Oaz1 (wild-type and 4res) and ODC-2xHa signals normalized to Cdc11 levels. Levels are given relative to, respectively, Oaz1 and ODC levels obtained with cells expressing wild-type Oaz1 in the absence of spd, which were set to 100%. Error bars, s.d.; n = 2. Significance values were calculated by paired T test comparing ODC levels to those with wild-type Oaz1 and without spd; P ≤ 0.05 (*) and P ≤ 0.01 (**).
(B) Western blot analysis of the steady state levels of ODC-2xHa and Oaz1-2xHa in a spe1-Δ oaz1-∆ strain in the presence of 5 mM DFMO. The plasmid-encoded ODC gene (SPE1) was expressed from its own promoter, whereas OAZ1-if(wt) was expressed from the copper-inducible PCUP1 promoter. CuSO4 was added to the medium as indicated. The last lane shows ODC and Oaz1 levels in cells grown without CuSO4 but with 50 µM spd showing decreased ODC levels compared to other lanes in spite of similar Oaz1 levels.
(C) In vitro degradation of ODC was assayed with 0.06 µg of 26S proteasome (1.6 nM) and 100 ng of ODC-Oaz1 heterodimer (80 nM) in a volume of 15 µL and varying concentrations of either spd or spermine (spm) as indicated. The graph shows the quantification of ODC-2xHa signals. Error bars, s.d.; n = 3. Signficance values were calculated by paired T test comparing ODC levels to the samples incubated for 60 min without spd; P ≤ 0.05 (*) and P ≤ 0.01 (**).