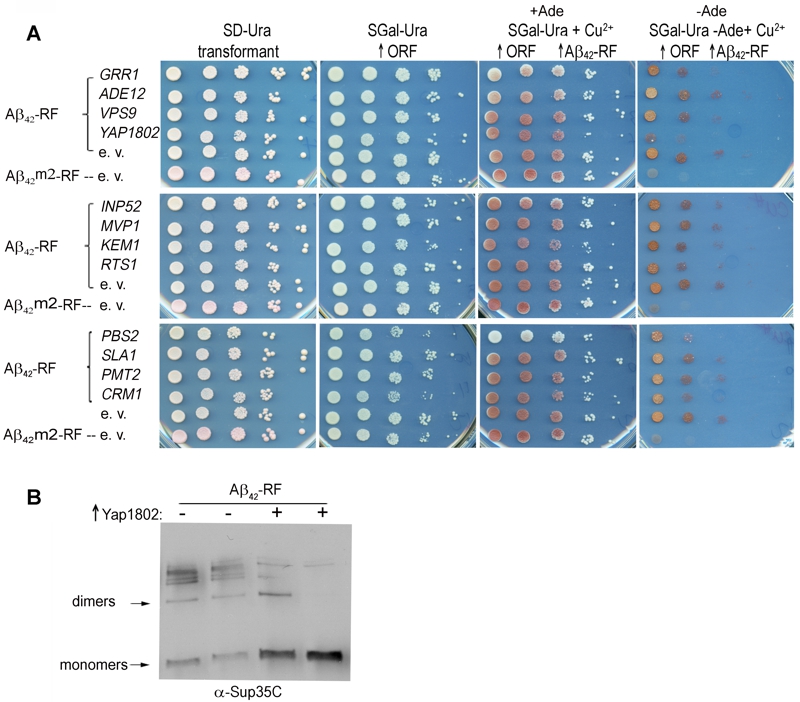
FIGURE 1: Effect of genetic modifiers of toxic HDEL-Aβ42 on Aβ42-RF oligomer formation and Aβ42-MRF aggregates into toxic SDS-stable oligomers in yeast.
(A) Overexpressed Yap1802 but not other previously described [24] HDEL-Aβ42 modifiers dramatically enhanced the translational termination factor activity of Aβ42-RF. Aβ42-RF expressing cells (CUP1::Aβ42-RF) lacking chromosomal SUP35 were transformed with a plasmid carrying the indicated gene under control of the GAL1 promoter. Ten-fold serial dilutions of transformants are shown on plasmid selective non-inducing media (SD-Ura); galactose plasmid selective media (SGal-Ura) to turn on each gene (↑ORF); +Ade medium containing copper to overexpress Aβ42-RF (↑ORF ↑Aβ42-RF); the identical -Ade medium SGal-Ura-Ade+Cu2+ (-Ade ↑ORF ↑Aβ42-RF) to determine translational termination factor activity as read-through of the ade1-14 nonsense mutant. The Aβ42-RF overexpressed in cells was shown to have reduced translational termination factor activity as cells grew on -Ade due to aggregation of the fusion protein into small oligomers (Aβ42-RF-e. v.). However, the translation termination factor activity was retained in yeast cells overexpressing Aβ42m2-RF (Aβ42 aggregation-deficient mutant) or YAP1802, due to the absence of oligomer formation, resulting in reduced growth on -Ade (Aβ42m2-RF-e. v.).
(B) Yap1802 suppression of Aβ42-RF oligomerization by immunoblot analysis. Total cell lysates were prepared from 2 independent transformants of an Aβ42-RF strain carrying YAP1802 (+) or an empty vector (-) plasmid. Both Aβ42-RF and YAP1802 were overexpressed (SGal-Ura-Ade+Cu2+) prior to lysis. Immunoblots were probed with anti-Sup35 RF to evaluate the level of oligomers and monomers. ↑Yap1802 indicates overexpression of YAP1802. The identification of bands as Aβ42-RF dimers and monomers was determined by the estimated sizes of the bands in the immunoblot.