Back to article: The ubiquitin-conjugating enzyme, Ubc1, indirectly regulates SNF1 kinase activity via Forkhead-dependent transcription
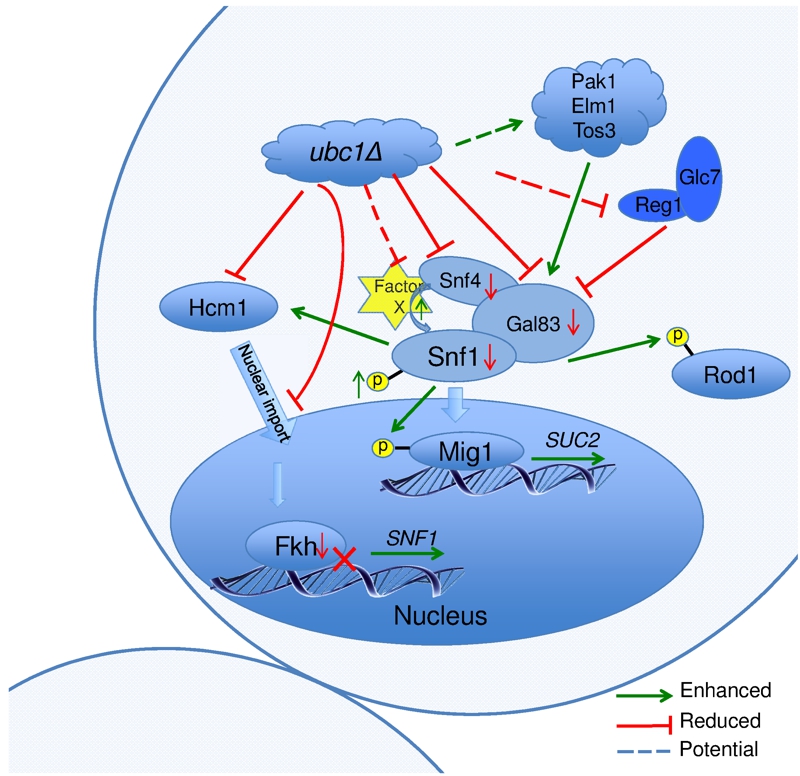
FIGURE 8: Schematic model of Ubc1-dependent mechanisms and their potential targets impacting SNF1 kinase activity.
In the ubc1Δ strain, the Hcm1 protein fails to shuttle to the nucleus in a cell cycle dependent manner. The mechanisms is unknown, yet may include the failure of Ubc1 to degrade a cytosolic Hcm1 tether, or to provide an Ub-mediated import signal. The lack of nuclear Hcm1 results in impaired expression of FKH1/2 genes, which required Hcm1 for expression. The decrease in Fkh1/2 protein in turn impedes the expression, and subsequent protein abundance, of Snf1. The Snf1 protein present, however, retained its functional ability to target cytosolic (Rod1) and nuclear (Mig1) proteins for phosphorylation, and itself be phosphorylated and translocated in response to activating conditions. There are enhanced allosteric associations between Snf1 and the regulatory Snf4 subunit in the absence of Ubc1 function, again by an unknown mechanism that may involve the removal of a moiety causing steric hindrance, Factor X. No obvious candidate protein is known that associates with the activated complex that would be stabilized by a loss of E2 activity.