Back to article: The copper transport-associated protein Ctr4 can form prion-like epigenetic determinants in Schizosaccharomyces pombe
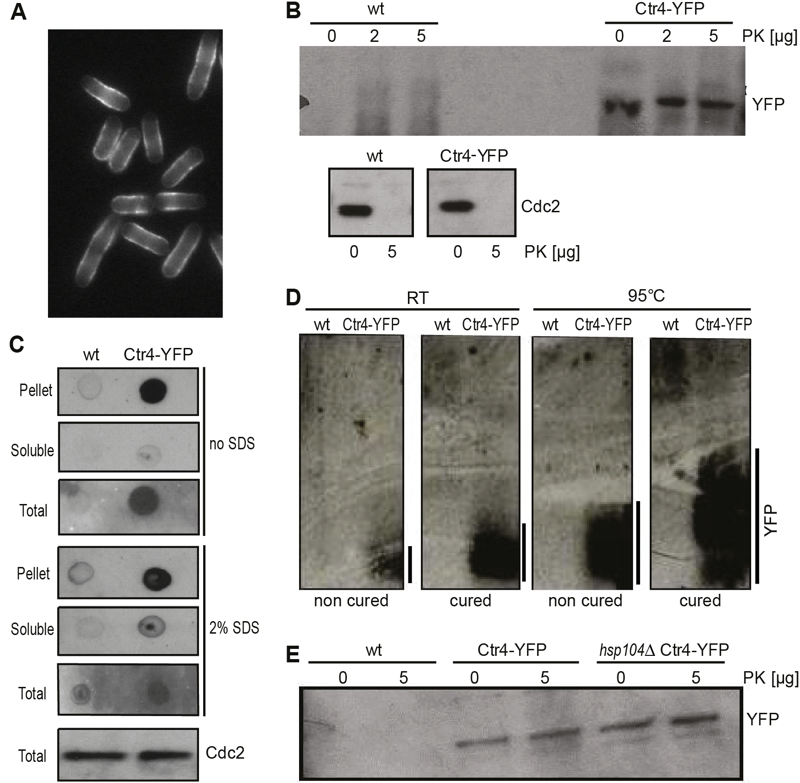
FIGURE 3: Ctr4 exhibits properties consistent with prion formation.
(A) Fluorescence micrograph of cells overexpressing Ctr4-YFP, showing localization and aggregation of Ctr4 at cell periphery.
(B) Extracts from wild-type (wt) cells, expressing native Ctr4, and cells overexpressing Ctr4-YFP, treated without (0) or with 2 or 5 µg proteinase K (PK) were run by SDS-PAGE followed by western blotting using anti-GFP and anti-Cdc2 antibodies to detect Ctr4 and Cdc2 (loading control), respectively.
(C) Dot plots with extracts from wild-type (wt) cells and cells overexpressing Ctr4-YFP using anti-GFP antibodies to detect YFP. Results for pellet, soluble and total cell fractions are shown as indicated, both with and without pre-treatment of the nitrocellulose membranes with 2% SDS. Bottom: SDS-PAGE of the same extracts to detect Cdc2 as a loading control.
(D) SDD-AGE gels of samples treated at room temperature (RT) and at 95°C, both with and without curing with GdnHCl as indicated. Overexpressed Ctr4-YFP forms high-molecular weight protein aggregates (right lanes); heat treatment and curing did not abolish the high-molecular weight species of Ctr4, but led to a larger range in aggregate sizes.
(E) Extracts from wild-type (wt) cells, cells overexpressing Ctr4-YFP and hsp104 deletion cells overexpressing Ctr4-YFP, treated without or with 5 µg PK were run by SDS-PAGE followed by western blotting using anti-GFP antibody to detect YFP.