Back to article: A novel basolateral type IV secretion model for the CagA oncoprotein of Helicobacter pylori
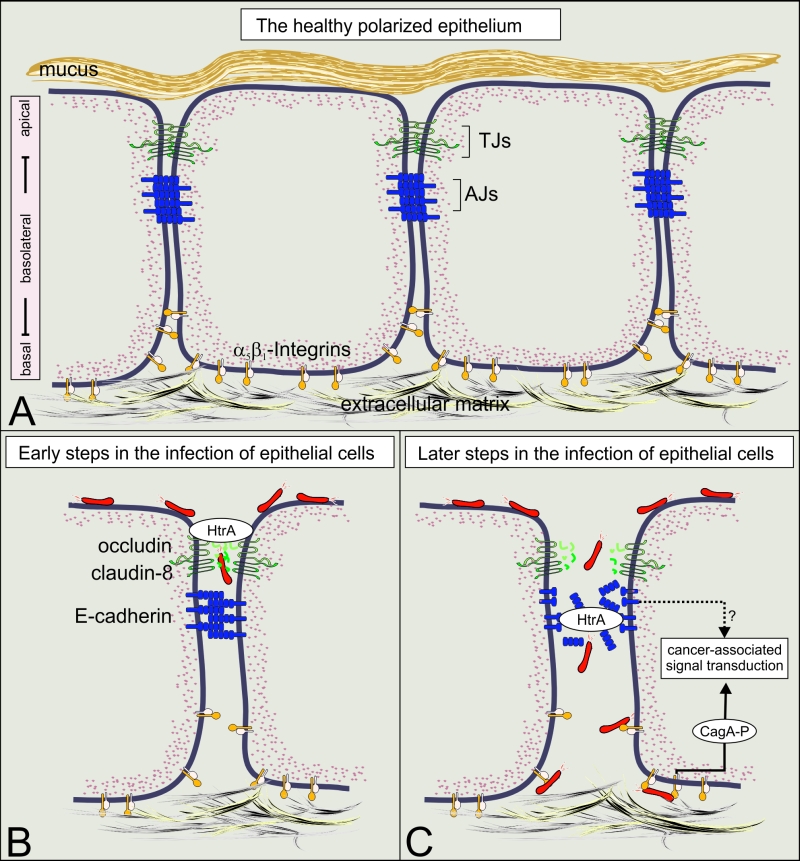
FIGURE 1: Translocation and phosphorylation of CagA in polarized cells require HtrA protease activity. (A) A model of the healthy polarized gastric epithelium. The surface of the stomach is protected by the gastric mucus. Polarization of the underlying epithelial cells is mainly established by intercellular adhesion complexes, including tight junctions (TJs) and adherence junctions (AJs). The TJs are directly located above AJs and mark the transition from the apical domain to the basolateral domain. The expression of the T4SS receptor integrin-β1 occurs at basolateral and basal domains of polarized epithelial cells and is crucially important for the adherence of cells to the extracellular matrix. (B) The current data indicate that H. pylori uses a paracellular transmigration route to reach integrin-b1 at basolateral surfaces as indicated. For this purpose, secreted HtrA targets specific host cell factors in the TJs (occludin and claudin-8) at early phases of infection. (C) At later phases of infection, E-cadherin-based AJs are disrupted, allowing the contact between the T4SS and integrin-β1 and subsequently the delivery of CagA into the cytoplasm of host cells. Upon CagA translocation, CagA is tyrosine-phosphorylated and hijacks host cell signaling cascades which are implicated in gastric carcinogenesis.