Reviews:
Microbial Cell, Vol. 10, No. 10, pp. 204 - 216; doi: 10.15698/mic2023.10.805
Phospholipases A and Lysophospholipases in protozoan parasites
1 Université de Bordeaux, Microbiologie Fondamentale et Pathogénicité, CNRS UMR 5234, Bordeaux, France.
Keywords: phospholipases, lysophospholipases, protozoan, host-pathogen interactions, virulence factors, metabolism.
Received originally: 15/05/2023 Received in revised form: 25/08/2023
Accepted: 05/09/2023
Published: 02/10/2023
Correspondence:
Loïc Rivière, Université de Bordeaux, Microbiologie Fondamentale et Pathogénicité, CNRS UMR 5234, Bordeaux, France; loic.riviere@u-bordeaux.fr
Conflict of interest statement:
The authors declare no competing interests.
Please cite this article as: Perrine Hervé, Sarah Monic, Frédéric Bringaud and Loïc Rivière (2023). Phospholipases A and Lysophospholipases in protozoan parasites. Microbial Cell 10(10): 204-216. doi: 10.15698/mic2023.10.805
Abstract
Phospholipases (PLs) and Lysophospholipases (LysoPLs) are a diverse group of esterases responsible for phos-pholipid or lysophospholipid hydrolysis. They are involved in several biological processes, including lipid catabolism, modulation of the immune response and membrane maintenance. PLs are classified depending on their site of hydrolysis as PLA1, PLA2, PLC and PLD. In many pathogenic microorganisms, from bacteria to fungi, PLAs and LysoPLs have been described as critical virulence and/or pathogenicity factors. In protozoan parasites, a group containing major human and animal pathogens, growing literature show that PLAs and LysoPLs are also involved in the host infection. Their ubiquitous presence and role in host-pathogen interactions make them particularly interesting to study. In this review, we summarize the literature on PLAs and LysoPLs in several protozoan parasites of medical relevance, and discuss the growing interest for them as potential drug and vaccine targets.
INTRODUCTION
Phospholipases (PLs) and lysophospholipases (LysoPLs) are a widespread and complex group of lipolytic enzymes responsible for the hydrolysis of phospholipids and lysophospholipids, respectively. They are classified as A1, A2, C and D according to their site of cleavage (Fig. 1) [1]. These enzymes are involved in several biological processes including modulation of the immune response, cell signaling, membrane remodeling and lipid metabolism [2][3]. Some of them, mainly phospholipases A (PLAs) and LysoPLs, play important roles in the virulence and pathogenicity of several groups of pathogens such as bacteria (Pseudomonas, Ricktesia) and fungi (Candida) [4][5]. PLAs are also major toxins of venomous species [6]. For detailed information regarding PLs, please refer to Aloulou et al. [1].
–
Protozoans form a group of eukaryotic unicellular organisms that contain parasites responsible for severe and even fatal diseases worldwide. Their modes of transmission are diverse and include vectors (insects, gasteropods, annelids, etc.), contaminated food, water or soil, congenital transmission, blood transfusion or organ transplantation. Some of the diseases they cause are especially prevalent in developing countries due to lower standards of living and an advantageous climate for the presence of vectors. However, migration and climate change could favor the spread of these parasites globally. While some parasites such as the Apicomplexan Plasmodium spp. and Toxoplasma are heavily studied, others remain neglected despite the high risks they represent for susceptible populations [7][8].
–
The importance of PLAs and LysoPLs for virulence in pathogenic species and their ability to manipulate the host immune response make them particularly interesting in the context of host – parasite interaction. Thanks to the sequencing of their genomes and the availability of many genomic resources, we know that most protozoan para-sites likely possess several PLA genes (https://veupathdb.org). However, recent studies are particularly focused on apicomplexan parasites, neglecting other major protozoan pathogens. Some characterized PLAs in pathogenic protozoan seem to be involved in the survival of the parasite inside its host and the establishment of an infection.
–
In this review, we focus on the different PLAs and LysoPLs characterized in several protozoan parasites of medical interest. We also discuss the relevance of these PLAs as potential drug and vaccine targets. The different parasites and enzymes described in this review are summarized in Table 1 at the end of the conclusion.
–
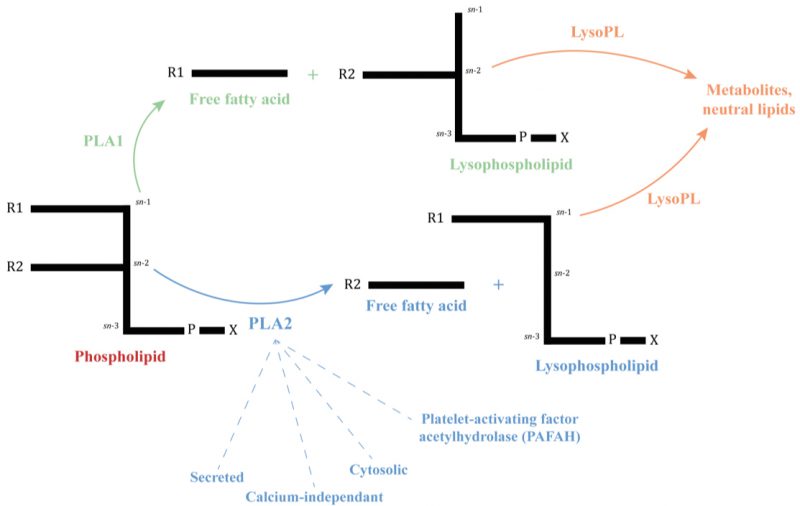 |
FIGURE 1: Simplified phospholipid structure and the site of cleavage of Phospholipases A and Lysophospholipases. A phospholipid consists of a glycerol-3-phosphate esterified with nonpolar fatty acids at its sn-1 (R1) and sn-2 (R2) positions and a polar headgroup (X = choline, ethanolamine, serine…) at its phosphoryl group. Phospholipases A are named A1 or A2 depending on their site on cleavage and the resulting molecules, indicated by arrows. Lysophospholipases act on lysophospholipids previously hydrolyzed by a PLA [1]. -
|
APICOMPLEXAN
Infections by Apicomplexan parasites represent huge health burdens worldwide. These parasites are characterized by the presence of the apicoplast, a plastid-like organelle which holds the lipid biosynthesis pathways [7].
–
Toxoplasma gondii
Toxoplasma gondii is estimated to chronically infect one third of the world’s population. This parasite can infect a wide range of warm-blooded animals, but its definitive hosts are felines. Although most infections are asymptomatic, congenital transmission or reactivation of latent disease in immunocompromised individuals such as AIDS, cancer or organ transplant patients can cause severe toxoplasmosis and represent significant public health burdens. Transmission usually occurs by ingestion of parasites or from an infected mother to the fetus. Toxoplasma parasites are present in three forms: an environment-resistant oocyst, a fast-replicating tachyzoite and a latent tissue cyst containing bradyzoites. Toxoplasma tachyzoites can replicate in a variety of nucleated cell types, while the chronic-stage bradyzoites persist as cysts within neurons or muscles [9].
–
Several PLAs of Toxoplasma have been characterized, including some important for the survival of the parasite in its host. TgLCAT (lecithin:cholesterol acyltransferase) produces cholesteryl esters through the transfer of phospholipid acyl groups (Table 1). The enzyme possesses both PLA2 and cholesteryl esterase activities and uses phosphatidylcholine (PC) as a substrate. TgLCAT is stored in dense granules and localizes to both the parasitophorous vacuole (PV) and plasma membrane following secretion from the dense granules. In a first study, parasites deficient for TgLCAT displayed a delay in replication and egress, suggesting a role in lipid membrane remodeling of both the parasite during replication and host cell during parasite escape. Mice infected with TgLCAT-null mutants showed reduced virulence, while parasites overexpressing TgLCAT were more virulent than the parental strain and resulted in earlier deaths [10]. In a later study using new strains, the authors confirmed its role in egress but showed that it has no contribution to replication in vitro or virulence in vivo [11].
–
Other PLA2s described include patatin-like phospholipases (PLPs), Ca2+-independent PLA2s. Three PLPs are known in Toxoplasma parasites. TgPL1 reduces the level of nitric oxide in activated macrophages to prevent its own degradation (Table 1) [12]. The enzyme lacks the catalytic serine and PLA2 activity, but the presence of other catalytic residues suggests a role in binding phospholipids or as a coenzyme for other PLA2 enzymes [13]. Mice infected with TgPL1-null mutants showed a greater resistance to toxoplasmic encephalitis and a change in cytokine levels during the chronic infection. The authors also observed a defect in bradyzoite-to-tachyzoite reactivation. The protein moves from within the parasite to the cyst wall and PV during tachyzoite-to-bradyzoite differentiation. Overall, TgPL1 seems to play a role in the maintenance of the infection in the host and toxoplasmosis encephalitis by altering cytokine production in the chronic phase [14].
–
A second PLP, TgPL2, is located at the apicoplast. This enzyme appeared essential for apicoplast maintenance and lipid homeostasis (Table 1). In parasites deficient for TgPL2 or with a mutated catalytic serine, the apicoplast was lost or became abnormal, leading to an impaired growth in vitro [15].
–
Ca2+-independent PLA2 activity associated with Ca2+– independent PLA1 activity was described in 2000, but it is unknown if one or several proteins were responsible for these activities [16].
–
Finally, PLA2 activity possibly linked to host cell invasion was first reported in 1989, when it was shown that incubation of the parasite with exogenous PLA2 from cobra (Naja mossambica) resulted in an increase of penetration in human fibroblasts. Meanwhile, PLA2 inhibition by 4-p-bromophenacyl bromide (4-BPB) decreased cell invasion, although the authors cautioned that 4-BPB seems to have a general toxic effect on parasites. Use of another PLA2 inhibitor, dihydroguaiaretic acid, also resulted in a decreased host cell invasion [17]. PLA2 activity could trigger arachidonic acid (AA) production from host cell phospholipids, which would alter the host cell membrane and facilitate parasite entry [18]. Incubation of extracellular Toxoplasma with exogenous PLA2 increased the presence of the rhoptry protein ROP1 in supernatant fractions, raising the possibility of a role of PLA2 in rhoptry protein secretion [19][20]. Based on these results, another team confirmed the role of 4-BPB on THP1 monocyte cells. Furthermore, IFN-y treatment caused a reduction in host cell invasion, but whether that effect was direct or not on PLA is unclear [21][22]. A PLP contributing to host cell invasion was recently reported. TgPL3 is located at the apical cap of the parasite. ΔTgPL3 parasites showed defects in invasion and rhoptry secretion; the authors hypothesize that TgPL3 has a role in either the mechanical release of rhoptry or in the signaling pathway leading to rhoptry release (Table 1). Mutation of the catalytic serine (S1409A parasites) also suppressed enzyme activity. Mice vaccination with ΔTgPL3 or S1409A parasites protected them against a subsequent infection with a lethal dose of Toxoplasma parasites [23].
–
Plasmodium sp.
Malaria is the most common and deadliest human parasitic disease, and one of the most prevalent human infectious diseases. In 2021, 247 million cases and 619,000 deaths were reported [24]. 90% of deaths occur in Africa and mainly affect children under 5 and pregnant women. Parasites are transmitted by female mosquitoes of the Anopheles genus. Most cases and deaths are attributed to P. falciparum, but 4 other species of Plasmodium are also responsible for human infections: P. vivax, P. ovale, P. malariae and P. knowlesi. Parasites continuously evolve inside their hosts: the sporozoite insect form, once injected into a human, turns into a merozoites in hepatocytes. Merozoites are released into the bloodstream where they invade red blood cells (RBCs) and produce gametocytes. Mosquitoes ingest these gametocytes during a blood meal [25].
–
Cells infected by Plasmodium provide lipid precursors to the parasites, which are necessary for de novo synthesis of phospholipids and neutral lipids. These lipids are used for membrane maintenance and remodelling [26]. The presence of (lyso)phospholipases is therefore essential for maintenance of Plasmodium inside their hosts. More than 20 potential genes were identified in the genome of Plasmodium parasites [26][27]. It was first shown that use of gentamicin and amikacin, aminoglycosides which bind to the 30S subunit of ribosomes, but are also known as PLA1 and PLA2 inhibitors, repressed P. falciparum growth in pretreated erythrocytes [28]. These enzymes could also be involved in the alteration of merozoite membrane phospholipid organization in the RBCs [29]. Furthermore, upon P. falciparum infection, both PLA2 and LysoPL activities were detected in human erythrocytes. LysoPL activity greatly increased in infected RBCs compared to non-infected ones and was much higher than PLA2 activity, suggesting that hydrolysis by LysoPLs is an important metabolic pathway for control of lysophospholipid levels. The authors never detected PLA2 activity from erythrocytes in uninfected conditions, but the origin of this activity (parasitic or infected RBCs) is unclear. PLA2 activity was inhibited in vitro by the antimalarial drugs chloroquine, quinine and artemether and LysoPL activity was inhibited by quinacrine and quinine, but the IC50 values for these inhibitions appear high and could be non-specific inhibitions due to high concentrations of the drugs. LysoPL activity was also inhibited by the sulfhydryl reagents p-hydroxymercuribenzoate and thimerosal, with IC50 values in much lower ranges [30][31]. Later on, it was shown that P. falciparum parasites internalize host RBC peroxiredoxin 6 (PRDX6), an enzyme with PLA2 activity. The authors found that this host enzyme is used by the parasite for repair of lipid-peroxidation damages in P. falciparum blood stages and is essential for hemoglobin transport to the parasite food vacuole. Use of PLA2 inhibitors binding to PRDX6, such as methyl arachidonyl fluorophosphonate (MAFP), ATK and Daraplabid, blocked parasite growth at the trophozoite stage. Use of Daraplabid on WT mice arrested model rodent pathogen P. yoelii development, but had no effect in transgenic prd6x-/- mice, demonstrating a possible use of PRDX6 as a host drug target for Plasmodium infection control [32]. The PLA2 activity detected in infected RBCs could therefore be coming from the host and not Plasmodium [30][32]. LysoPC from the host is also important for P. falciparum differentiation during infection. Restriction of the lipid, which occurs during malaria infection, led in vitro to metabolic adaptation of parasites, initiating sexual commitment and formation of gametocytes [33].
–
Several PLAs and LysoPLs have been identified in Plasmodium species. PfLPL1 is a LysoPL localized in the endoplasmic reticulum (ER) at the early stages. After erythrocyte invasion, the enzyme moves to vesicular structures that are later incorporated into lipid bodies (Table 1). These bodies are found next to the food vacuole, where is stored hemozoin, the neutralized form of the toxic by-product heme. PfLPL1 generates lipids necessary for hemozoin formation; downregulation of the enzyme resulted in disrupted lipid homeostasis and reduced levels of neutral lipids essential for conversion of toxic heme to hemozoin [34]. Another LysoPL from P. falciparum, PfLPL20, hydrolyzes lysophosphatidylcholine (LysoPC) from the host to generate metabolites that can be used by the parasite for its growth. Like PfLPL1, PfLPL20 localizes to vesicular structures that later fuse in lipid bodies [35].
–
Two PLs were characterized in the rodent malaria model pathogen, P. berghei. First, PbPL is involved in sporozoite migration, localizes at their surface and possesses both PLA2 and LCAT activity (Table 1). Infection of mice with null-mutant sporozoites through mosquito bite resulted in a significant decrease in liver infectivity. Aa decrease in epithelial cell layers crossing was observed in PbPL knock-out parasites. Furthermore, an hemolytic assay showed that heterologously expressed PbPL was able to damage cell membranes, which the authors hypothesize could be caused by direct hydrolysis of membrane PC or through the production of LysoPC species, which possess membrane lysis activities [36]. In infected hepatocytes, PbPL is located at the PV membrane (PVM) where it plays a role in its disruption; PbPL-deficient parasites showed a defect in merozoite release from hepatocytes and oocysts [37]. This inefficient merozoite release was also reported in PbPL’s ortholog in P. falciparum, PfLCAT. Lipidomics showed no significant changes in PC or LysoPC levels in PfLCAT-null mutants, but an accumulation of phosphatidylserine during egress was observed. PfLCAT-mutants also showed an inefficient egress in asexual blood stages (Table 1) [38]. A second enzyme from P. berghei, PbPLA1, is a phosphatidic acid preferring PLA1 (Table 1). The cytoplasmic enzyme is not expressed in sporozoites and is not essential in both mice infection and during mosquito stages. Pla1– parasites, like PbPL-deficient parasites, showed a defect in merozoite release from hepatocytes [39].
–
Just as Toxoplasma, Plasmodium species possess PLPs. PfPATPL1 is expressed in the cytosol of asexual blood stage and gametocyte stages of P. falciparum. Depletion of the enzyme resulted in reduced efficiency of rounding up, egress of gametes after gametocyte activation as well as exflagellation of male gametes. Null-mutants for PfPATPL1 showed an impaired translocation of the perforin-like protein PfPLP2 to the membrane periphery. This translocation is necessary for destabilization of vacuolar and RBC membranes, allowing egress of gametes [40]. In a later study, another group described an effect of the PLP, which they re-named PNPLA1, in gametocyte induction rather than a deficiency in gametocytogenesis (Table 1) [41].
–
Cryptosporidium
Cryptosporidiosis is a major diarrheal disease in young children and immunocompromised individuals worldwide. Food and water contaminated by feces or contact with infected animals and people are responsible for the transmission of parasites. Cryptosprodium parvum and hominis are the most prevalent species found during human infections. Cryptosporidium can complete its life cycle in a single host, and both asexual and sexual stages are found in the intestine of infected humans. The parasite does not possess an apicoplast or mitochondrial DNA and relies on its host metabolism to survive. After ingestion of oocysts, released sporozoites invade the small intestine and develop inside epithelial cells in an extracytoplasmic niche, where they maintain the infection [42].
–
Currently, not a single PLA/LysoPL gene is characterized in Cryptosporidium. However, secreted PLA2 (sPLA2) activity was observed in parasite lysate and could be involved in the invasion of the host enterocytes. Inhibition of PLA activity by 4-BPB or anti-sPLA2 antibodies resulted in a significant reduction in parasite reproduction in human enterocyte cell lines. Furthermore, treatment of enterocytes or C. parvum sporozoite with sPLA2 derived from cobra (Naja naja) venom before in vitro infection enhanced the number of intracellular parasites [43].
KINETOPLASTIDS
Trypanosomatids include major pathogenic parasites responsible for neglected tropical diseases. These parasites are part of the Kinetoplastid group, organisms which possess within their single mitochondrion a concatenated and compacted network of circular DNA named kinetoplast [8].
–
African Trypanosomes
Human and Animal African trypanosomiasis (HAT/AAT) are neglected tropical diseases caused by difference species of Trypanosoma. Two subspecies of Trypanosoma brucei, namely T. b. gambiense and T. b. rhodesiense, cause HAT or sleeping sickness. T. b. brucei, T. vivax and T. congolense are responsible for Nagana or AAT. The incidence of HAT has greatly decreased and WHO estimates an interruption of its transmission by 2030, but 70 million people remain at risk for the disease [44]. AAT cases are still very high, annually causing the death of 3 million livestock and losses of billions of US dollars in Africa. Parasites are transmitted by tsetse flies (Glossina) [44][45]. African trypanosomes have a complex life cycle inside their hosts. The bloodstream mammalian form differentiates into procyclics when a tsetse fly takes a blood meal. In the insect, procyclics differentiate into epimastigotes and finally infective metacyclics, which are transmitted to mammals during a blood meal. In humans, infection occurs in two phases: an early hemolymphatic stage (fever, adenopathy…) and a late encephalitic stage in the CNS (neurologic troubles, biological clock disorders…) which eventually leads to death without adequate treatment. [44]. In animals, anemia and cachexia are the main symptoms, but abortion, decrease of milk production and weight loss are also observed [46].
–
Higher levels of PLAs activities were first observed in T. brucei and T. congolense compared to the nonpathogenic T. lewisi species present in rodents [47]. This activity was more important in bloodstream forms [48] and increased with parasite burden in tissue fluids and blood plasma of T. brucei-infected rabbits [49]. Bloodstream forms also generated phospholipids from exogenous lysophospholipids; this activity may help the parasite acquire fatty acids for the synthesis of the variant surface glycoprotein (VSG) membrane [50]. Indeed, a GPI-specific PLA may be involved in fatty acid remodelling leading to the biosynthesis of glycosylphosphatidylinositol (GPI), which anchors the trypanosome VSG to the plasma membrane [51]. Lastly, LysoPLA1 and PLA1 activities were eluted together from T. brucei soluble protein fractions and were indissociable, suggesting that the parasite possesses an enzyme displaying both activities [52].
–
Two PLA1 genes have been characterized so far, both in T. brucei. TbPLA1 is a cytosolic enzyme displaying both PLA1 and LysoPLA1 activities and its preferred substrate is PC, resulting in the synthesis of LysoPC metabolites (Table 1) [53]. The enzyme levels and resulting metabolites were higher in the bloodstream forms compared to procyclics. Mutants for TbPLA1 were deficient in LysoPC synthesis. Homologues of this enzyme are found in the closely related T. congolense and T. vivax, but absent from more distant African trypanosomes or other Trypanosomatids. Its closest homologue is a putative PLA1 from Soladis glossinidius, a proteobacterium endosymbiont of the tsetse fly, suggesting that TbPLA1 may have been acquired through horizontal gene transfer [54].
–
TbLysoPLA is a recently characterized LysoPL. This secreted enzyme is localized in both the cytosol and glycosomes of bloodstream forms of T. brucei. TbLysoPLA showed PLA1 activity on non-natural phospholipids and PLA2 activity on natural phospholipids in vitro (Table 1). The enzyme acts on PC to produce LysoPC metabolites. TbLysoPLA is not essential for parasite survival but is probably highly immunogenic, since antibodies directed against it were generated during mice infection with T. brucei gambiense [55][56].
–
PLA2 activity was also detected in African trypanosomes. In T. brucei bloodstream and procyclic forms, it may regulate Ca2+ entry in the parasite and catalyze AA release, which could be used to control eicosanoid acid production and cause changes in the host cell membrane [57][58]. Furthermore, AA is the precursor for prostaglandin E2 (PGE2), a lipidic inflammatory mediator involved in mammalian immune responses [59]. However, this AA release from phospholipids can be PLA2-independent through a sequential sn-1 deacylation followed with hydrolysis by LysoPLA [60].
–
As previously mentioned, GPI-PLA activity was thought to be involved in trypanosome VSG remodeling [51]. A protein with GPI-PLA2 activity mediating the GPI precursor fatty acid remodeling was identified in procyclic T. brucei parasites. Deletion of the enzyme resulted in the suppression of GPI-PLA2 activity (Table 1). However, this enzyme was only characterized in procyclic parasites; VSG remodeling is an essential feature of bloodstream parasites, and whether or not it is essential in this form and its definitive role remain to be studied. The authors also caution that procyclic and bloodstream form parasites may use different genes for this same activity. Moreover, the authors hypothesize that another gene studied in this paper may encode for a GPI-PLA1 protein or a GPI-PLA2 activity in bloodstream parasites, but did not show it [61].
–
Anemia is a major symptom of T. congolense infection. A PLA2 from the parasite may be directly or indirectly responsible for the hemolytic activity observed during the course of infection, but the enzyme(s) involved is/are unidentified [62][63][64][65][66].
–
Trypanosoma cruzi
Trypanosoma cruzi is responsible for Chagas disease or American trypanosomiasis. This anthropozoonosis is endemic in Latin America, although human migrations are leading to a global spread of the parasite. In endemic regions, the annual number of cases is estimated at 6 to 7 million, with many more at risk. T. cruzi is mainly transmitted by triatomine insects, also known as kissing bugs. The parasite has three main stages: a non-infective insect epimastigote, an infective trypomastigote and an intracellular replicating form inside mammals, the amastigote. The acute phase of infection is usually asymptomatic, with parasites invading and replicating in the peripheral circulation. Parasites can migrate to cardiac and digestive tissues where they contribute to the chronicity of the disease [67].
–
Phospholipases of T. cruzi are likely involved in global cellular lipid remodeling throughout the parasite’s life cycle and temperature acclimation [68]. Both PLA1 and PLA2 membrane-associated activities on anionic phospholipids were observed in epimastigotes and may also be implicated in differentiation from trypomastigotes to amastigotes [69][70]. Furthermore, the presence of exogenous PLA2 significantly increased association between T. cruzi and macrophages, but the origin of the enzyme was not specified. This association was reduced by use of PLA2 inhibitors such as 4-BPB, quinacrine and phentermine. A PLA2 could therefore be involved in macrophage invasion [71].
–
The previous observation in T. brucei that Ca2+ entry was regulated by generation of AA by PLA2 activity was also seen in T. cruzi amastigotes by addition of a PLA2 activator, melittin. In parallel, addition of the PLA2 inhibitor 3-(4-octadecyl)-benzoylacrylic acid (OBAA) decreased this influx [58].
–
TcPLA1 is the only PLA characterized in T. cruzi. It is responsible for PC degradation in autolysing parasites. The enzyme possesses both PLA1 and LysoPLA1 activities and its PLA1 activity is 20-fold higher in the infective trypomastigotes and amastigotes compared to non-infective epimastigotes [72]. In epimastigotes, TcPLA1 is found in the lysosome while in infective stages the activity of the enzyme is membrane-bound. Secretion of TcPLA1 was observed during differentiation from epimastigotes to trypomastigotes. In vitro, TcPLA1 generates DG, FFA and LPC, lipid secondary messengers that activate host cell protein kinase C which leads to an upregulation of parasite invasion [73]. Accumulation of bioactive products such as LysoPC could be controlled by the LysoPLA activity of the enzyme. Distinct levels of TcPLA1 activity were found between lethal and non-lethal strains of bloodstream trypomastigotes and use of antibodies directed against TcPLA1 reduced host cell invasion in vitro [74]
–
Leishmania spp.
Leishmaniasis comprises several zoonotic disease endemic in many countries of Latin America and Asia. More than 20 species of Leishmania are responsible for the different clinical manifestations of leishmaniasis. The two main clinical manifestations are visceral leishmaniasis and cutaneous leishmaniasis. Hundreds of millions are at risk for each disease in almost 100 endemic countries. 700,000 to 1 million cases are reported every year. Parasites are transmitted by female phlebotomine sandflies. Leishmania exists in two forms: the mammalian stage amastigote and the insect stage promastigote. After infection, promastigotes parasites are phagocytosed by host macrophages, where they differentiate into infective amastigotes and multiply inside a parasitophorous vacuole. Parasites can persist for decades and reactivate in immunocompromised patients [75].
–
A first report showed that infection of macrophages by L. amazonensis, causative agent of cutaneous leishmaniasis, resulted in a significant increase of LysoPC in the host cell. LysoPC and AA are products of PLA2 activity on PC; production of PGE2 from AA is known to exacerbate Leishmania infection in macrophages [76][77]. However, the origin of this activity (parasite or macrophage) was not specified [76]. PLA2 activity was detected in the supernatant and lysate of L. amazonensis. Treatment of amastigotes with PLA2 from Crotalus durissus collilineatus snake increased the growth of parasites within macrophages. Infection of BALB/c mice after promastigote treatment with PLA2 led to an increase in lesion size and larger regions of necrosis, as well as a higher density of inflammatory infiltrate. PLA2 activity inhibited IL-2 levels, a cytokine associated with a protective Th1 response and PGE2 generation. PLA2 activity in Leishmania parasites thus may be a mediator of cutaneous leishmaniasis [78]. In parallel, addition of the PLA2 inhibitors bromoenol lactone (BEL or MAFP) affected the survival of L. (L.) amazonensis promastigotes in culture. Treatment of intracellular L. (L.) amazonensis amastigotes with the same inhibitors resulted in decreased macrophage parasitism. Finally, BALB/c mice infected with L. (L.) amazonensis and treated with BEL presented a reduction of lesion size after 6 weeks of infection compared to non-treated infected mice. This decrease was associated with decreased skin parasitism [79].
–
Similarly, to T. cruzi, addition of melittin increased Ca2+ influx in in L. donovani promastigotes. Inhibition by OBAA reduced this influx [58].
–
Leishamania parasites possess in their genome a LmHydrolPAFAH gene (platelet-activating factor-acetylhydrolase) encoding a Ca2+-independent PLA2 which hydrolyzes platelet-activating factors (PAF), phosphoglycerides involved in inflammation (Table 1). The enzyme is found in the ER of promastigotes and amastigotes. Null-mutants for the enzyme showed no phenotype in vitro but a reduced virulence was observed in L. major-infected BALB/c mice. LmPAFAH may regulate levels of host platelet-activating factor (PAF), which could inhibit Leishmania survival in macrophages by NO activation. It may also be involved in mediation of levels of LysoPC to facilitate parasite survival [80]. The PAFAH protein from L. major, along with its ortholog in T. cruzi and T. brucei, is therefore an interesting drug and vaccine target [81].
–
LbPLA1 from L. braziliensis is responsible for PC hydrolysis in infective amastigotes and insect promastigotes. The purified recombinant protein exhibited PLA1 activity. The enzyme had a higher activity in amastigotes [82].
–
LdLip3 is a secreted lipase from L. donovani. The enzyme, found in both amastigote and promastigote forms, could act on host fatty acids in order to synthesize lipids for parasite growth and survival, as well as tissue damage associated with leishmaniasis (Table 1) [83].
OTHER PROTOZOAN PARASITES OF MEDICAL RELEVANCE
Beside the more popular Apicomplexan and Trypanosomatids, some protozoan parasites such as amoebas or other flagellates cause severe disease worldwide.
–
Entamoeba histolytica
Intestinal amebiasis caused by Entamoeba histolytica is a prevalent disease in developing countries with lower standards of sanitation. With 100 million cases each year, it is the fourth highest infectious parasite. 90% of infections remain asymptomatic. Two stages have been described: an active pathogenic trophozoite and a disseminating environmental amebic cyst. After ingestion of cysts through contaminated stools, released trophozoites invade the intestine and colonize the colon. In symptomatic cases, parasites interact with the epithelial cells and cause tissue damages [84].
–
It was first shown that antagonists of PLAs such as the PC analog Rosenthal’s inhibitor and hydrocortisone were able to inhibit the cytolytic effects of trophozoite parasites in vitro, suggesting the involvement of PLAs in host cells lysis. [85]. The authors raised the hypothesis that Entamoeba PLA could directly act on the membrane of the target cell, or indirectly by the generation of cytotoxic lysocompounds. A more speculated argument could be the insertion of PLA in membranes, resulting in the activation of intracellular host PL and inducing autolysis in the presence of extracellular Ca2+ [85]. The same team also described Ca2+-dependent and Ca2+-independent PLAs. The first one was associated with membrane fractions while the second was mainly found in soluble fractions. A link was then established between these activities and cytolytic effects on host cells [86]. Hemolytic activity of E. histolytica was associated with a vesicular subcellular fraction called P30 and most likely due to a PLA [87][88][89]. In this fraction, both PLA1 and PLA2 as well as LysoPLA activities were identified [90]. Finally, it was observed that long-term axenic culture of Entamoeba caused a diminution of erythrophagocytosis, PLA2 and hemolytic activities. This was correlated with an impaired virulence of parasites [91]. The role of these PLAs in the virulence and pathogenicity of the parasite remains to be described. Although the genome of E. histolytica was sequenced in 2005 [92], no PLA-encoding gene has been characterized yet.
–
Naegleria fowleri
Primary amoebic meningoencephalitis by the brain-eating amoeba Naegleria fowleri is a deadly, fulminating disease with a 98% mortality rate. The parasite possesses three forms: a pathogenic trophozoite, a transitory flagellated form in the presence of water and an environment-resistant cyst. After the parasite enters its host, most commonly through the nose when swimming, replicating trophozoites migrate to the olfactory bulb, where they enter the brain. Once in the CNS, the parasites proliferate and destroy tissue, ultimately causing death in 7 to 10 days [93].
–
In the early eighties it was shown that PLA, LysoPL and sphingomyelinase activities were substantially increased in the virulent N. fowleri cell line compared to non-pathogenic Naegleria spp. These activities were associated with cells and cell-free culture media. Moreover, the authors showed evidence that lipolytic activities were released in the media of the virulent cell line but not in culture media of non-pathogenic attenuated cells [94]. In a later study, the authors showed that PL-enriched culture of pathogenic Naegleria induced the degradation of human phospholipids [95]. This secreted activity could be at least partially responsible for the lipolytic activity observed in primary amoebic meningoencephalitis [96]. A Ca2+-independent PLA2 associated with membrane fraction could play a role in the parasite metabolism [97]. However, no PLA gene has been characterized yet.
–
Giardia
With a prevalence up to 30% in developing countries and 300 million cases reported each year, giardiasis is one of the most common diarrheal disease worldwide. G. duodenalis, also known as G. intestinalis or G. lamblia, can infect all mammals. Infection occurs via the fecal-oral route, most commonly by ingestion of contaminated water. Giardia exists in two forms: a replicating trophozoite and a form of dissemination, the cyst. Once ingested cysts reach the stomach, the acidic pH and gastric enzymes induce an excystation. Parasites then migrate to the duodenum and differentiate into trophozoites. They adhere to intestinal epithelial cells where they multiply. Trophozoite can encyst back into quadrinucleated cysts, which are shed in the environment through the feces [98].
–
PLA activity has been detected in in vitro culture of Giardia [99][100]. Like for Entamoeba, PLA2 activity was found in the P30 subcellular fraction. Cell fractionation suggested that two isoforms of PL sensitive to Rosenthal’s inhibitor could be separated, one being membrane-bound and the other soluble [99]. The authors hypothesized that these activities could be responsible for the cytotoxicity of the parasite [99]. Interestingly, Giardia intestinalis is sensitive to the lipase inhibitor Tetrahydrolipstatin (Orlistat) in vitro, a drug which is also active on trypanosomes and Plasmodium. The target of Orlistat could be enzymes with a GXSXG motif, which is found in both PLA and fatty acid synthases [101].
–
Trichomonas vaginalis
Trichomoniasis is the most common non-viral sexually transmitted infection in the world, with more than 250 million cases per year. Trichomonas only exists as a trophozoite. After infection, the parasite colonizes the human lower urogenital tract and adhere to host genital epithelial cells. Men are more commonly asymptomatic than women, but both are carriers and can manifest symptoms. [102]. Several studies suggest the role of a PLA2 in the pathogenicity of this parasite. PLA2 activity was measured in the vaginal fluids of pregnant women, which correlated with Trichomonas infection [103]. A material found in the soluble lytic fraction of Trichomonas had hemolytic activity and was able to degrade PC with lysed profiles similar to those obtained with a PLA2, suggesting that this lipase activity is PLA2-like [104]. PLA activity was detected in a subcellular fraction of Trichomonas. An hemolytic activity was correlated with the fraction, which decreased with addition of Rosenthal’s inhibitor [105]. This observation is consistent with PLA1 and PLA2 activities from other parasites being involved in hemolysis [62][63][64][65][87][88][89]. Indeed, PLA1 and PLA2 activities associated with soluble and membrane-bound fractions could induce indirect and direct hemolytic activities [105]. A PLA2 was isolated from a subcellular fraction, but solid functional genomics proofs need to be added. This eluted fraction caused hemolysis and was inhibited by Rosenthal’s inhibitor [106]. The genome of T. vaginalis was sequenced in 2007 [107], but no PLA gene has been identified since then.
CONCLUSION
Phospholipases and lysophospholipases play a crucial role in the host-parasite interactions by performing a variety of functions. For the last few years, there has been a clear interest in the study of these enzymes, with the discovery of new genes and new functions paving the way for further investigations. Studies have revealed their importance in the development and maintenance of protozoan pathogens inside their host, notably in common processes such as the invasion, host damages, production of active compounds and scavenging of metabolites for parasite survival. As of now, no single protozoan PLA/LysoPL has been shown to be essential. This could be explained by the fact that these parasites possess not only one but an arsenal of PLs that could compensate for the loss of one. Their roles in the virulence of these pathogens make them potential targets for drugs, vaccine or diagnostic development as was demonstrated for several protozoan or even host PLAs [23][32][55][56][80][81]. The genome of protozoan parasites contains several different PL and LysoPL genes, but only a few have been characterized and studied yet, as summarized in Table 1, making their study a future necessity. Moreover, as we saw in this review there is, in some pathogens, a complete lack of knowledge at the gene level such as in intestinal parasites. It is crucial to continue deciphering the complexity of (lyso)phospholipases to improve our understanding of protozoan parasites and possibly eliminate them.
–
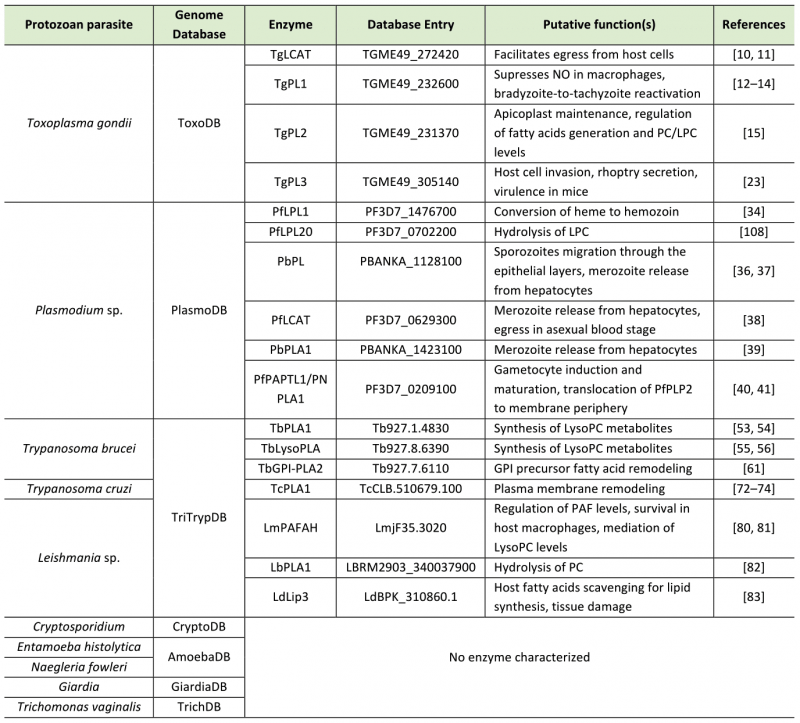
Table 1. Characterized PLAs and LysoPLs of protozoan parasites. |
 |
REFERENCES
- Aloulou A, Rahier R, Arhab Y, Noiriel A, and Abousalham A (2018). Phospholipases: An Overview: Methods and Protocols. In: Methods in molecular biology (Clifton, N.J.). pp 69–105. 10.1007/978-1-4939-8672-9_3
- Aoki J, Inoue A, Makide K, Saiki N, and Arai H (2007). Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 89(2): 197–204. 10.1016/j.biochi.2006.09.021
- Burke JE, and Dennis EA (2009). Phospholipase A2 structure/function, mechanism, and signaling1. Journal of Lipid Research. 50: S237–S242. 10.1194/jlr.R800033-JLR200
- Flores-Díaz M, Monturiol-Gross L, Naylor C, Alape-Girón A, and Flieger A (2016). Bacterial Sphingomyelinases and Phospholipases as Virulence Factors. Microbiol Mol Biol Rev. 80(3): 597–628. 10.1128/MMBR.00082-15
- Köhler GA, Brenot A, Haas-Stapleton E, Agabian N, Deva R, and Nigam S (2006). Phospholipase A2 and Phospholipase B Activities in Fungi. Biochimica et biophysica acta. 1761(11): 1391. 10.1016/j.bbalip.2006.09.011
- Kini RM (2006). Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J. 397(Pt 3): 377–387. 10.1042/BJ20060302
- Verhoef JMJ, Meissner M, and Kooij TWA Organelle Dynamics in Apicomplexan Parasites. mBio. 12(4): e01409-21. 10.1128/mBio.01409-21
- Stuart K, Brun R, Croft S, Fairlamb A, Gürtler RE, McKerrow J, Reed S, and Tarleton R (2008). Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 118(4): 1301–1310. 10.1172/JCI33945
- Matta SK, Rinkenberger N, Dunay IR, and Sibley LD (2021). Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol. 19(7): 467–480. 10.1038/s41579-021-00518-7
- Pszenny V, Ehrenman K, Romano JD, Kennard A, Schultz A, Roos DS, Grigg ME, Carruthers VB, and Coppens I (2016). A Lipolytic Lecithin:Cholesterol Acyltransferase Secreted by Toxoplasma Facilitates Parasite Replication and Egress. J Biol Chem. 291(8): 3725–3746. 10.1074/jbc.M115.671974
- Schultz AJ, and Carruthers VB (2018). Toxoplasma gondii LCAT Primarily Contributes to Tachyzoite Egress. mSphere. 3(1). 10.1128/mSphereDirect.00073-18
- Mordue DG, Scott-Weathers CF, Tobin CM, and Knoll LJ (2007). A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages: Isolation of Toxoplasma patatin-like protein. Molecular Microbiology. 63(2): 482–496. 10.1111/j.1365-2958.2006.05538.x
- Tobin Magle C, Pittman KJ, Moser LA, Boldon KM, Knoll LJ, and Urban JF (2014). A Toxoplasma Patatin-Like Protein Changes Localization and Alters the Cytokine Response during Toxoplasmic Encephalitis. Infection and Immunity. 82(2): 618–625. 10.1128/IAI.00444-13
- Tobin CM, and Knoll LJ (2012). A Patatin-Like Protein Protects Toxoplasma gondii from Degradation in a Nitric Oxide-Dependent Manner. Infection and Immunity. 80(1): 55–61. 10.1128/IAI.05543-11
- Lévêque MF, Berry L, Yamaryo-Botté Y, Nguyen HM, Galera M, Botté CY, and Besteiro S (2017). TgPL2, a patatin-like phospholipase domain-containing protein, is involved in the maintenance of apicoplast lipids homeostasis in Toxoplasma: A phospholipase important for apicoplast homeostasis. Molecular Microbiology. 105(1): 158–174. 10.1111/mmi.13694
- Cassaing S, Fauvel J, Bessières MH, Guy S, Séguéla JP, and Chap H (2000). Toxoplasma gondii secretes a calcium-independent phospholipase A(2). Int J Parasitol. 30(11): 1137–1142. 10.1016/s0020-7519(00)00101-6
- Saffer LD, Long Krug SA, and Schwartzman JD (1989). The role of phospholipase in host cell penetration by Toxoplasma gondii. Am J Trop Med Hyg. 40(2): 145–149. 10.4269/ajtmh.1989.40.145
- Thardin JF, M’Rini C, Beraud M, Vandaele J, Frisach MF, Bessieres MH, Seguela JP, and Pipy B (1993). Eicosanoid production by mouse peritoneal macrophages during Toxoplasma gondii penetration: role of parasite and host cell phospholipases. Infect Immun. 61(4): 1432–1441. 10.1128/iai.61.4.1432-1441.1993
- Saffer LD, and Schwartzman JD (1991). A soluble phospholipase of Toxoplasma gondii associated with host cell penetration. J Protozool. 38(5): 454–460. 10.1111/j.1550-7408.1991.tb04816.x
- Ossorio PN, Schwartzman JD, and Boothroyd JC (1992). A Toxoplasma gondii rhoptry protein associated with host cell penetration has unusual charge asymmetry. Mol Biochem Parasitol. 50(1): 1–15. 10.1016/0166-6851(92)90239-g
- Gómez Marín JE, Bonhomme A, Guenounou M, and Pinon JM (1996). Role of interferon-gamma against invasion by Toxoplasma gondii in a human monocytic cell line (THP1): involvement of the parasite’s secretory phospholipase A2. Cell Immunol. 169(2): 218–225. 10.1006/cimm.1996.0112
- Gomez-Marín JE, El’Btaouri H, Bonhomme A, Antonicelli F, Pezzella N, Burlet H, Aubert D, Villena I, Guenounou M, Haye B, and Pinon JM (2002). Involvement of secretory and cytosolic phospholipases A2 during infection of THP1 human monocytic cells with Toxoplasma gondii. Effect of interferon gamma. Parasitol Res. 88(3): 208–216. 10.1007/s00436-001-0525-z
- Wilson SK, Heckendorn J, Martorelli Di Genova B, Koch LL, Rooney PJ, Morrissette N, Lebrun M, and Knoll LJ (2020). A Toxoplasma gondii patatin-like phospholipase contributes to host cell invasion. PLoS Pathog. 16(7): e1008650. 10.1371/journal.ppat.1008650
- World Health Organization. (2022). World malaria report 2022. World Health Organization. https://apps.who.int/iris/handle/10665/365169. Licence: CC BY-NC-SA 3.0 IGO
- Cowman AF, Healer J, Marapana D, and Marsh K (2016). Malaria: Biology and Disease. Cell. 167(3): 610–624. 10.1016/j.cell.2016.07.055
- Flammersfeld A, Lang C, Flieger A, and Pradel G (2018). Phospholipases during membrane dynamics in malaria parasites. Int J Med Microbiol. 308(1): 129–141. 10.1016/j.ijmm.2017.09.015
- Elahi R, Ray WK, Dapper C, Dalal S, Helm RF, and Klemba M (2019). Functional annotation of serine hydrolases in the asexual erythrocytic stage of Plasmodium falciparum. Sci Rep. 9(1): 17532. 10.1038/s41598-019-54009-0
- Krugliak M, Waldman Z, and Ginsburg H (1987). Gentamicin and amikacin repress the growth of Plasmodium falciparum in culture, probably by inhibiting a parasite acid phospholipase. Life Sci. 40(13): 1253–1257. 10.1016/0024-3205(87)90581-9
- Joshi P, and Gupta CM (1988). Abnormal membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Br J Haematol. 68(2): 255–259. 10.1111/j.1365-2141.1988.tb06198.x
- Zidovetzki R, Sherman IW, and O’Brien L (1993). Inhibition of Plasmodium falciparum phospholipase A2 by chloroquine, quinine, and arteether. J Parasitol. 79(4): 565–570. 8331477
- Zidovetzki R, Sherman IW, Prudhomme J, and Crawford J (1994). Inhibition of Plasmodium falciparum lysophospholipase by anti-malarial drugs and sulphydryl reagents. Parasitology. 108 (Pt 3): 249–255. 10.1017/s0031182000076095
- Wagner MP, Formaglio P, Gorgette O, Dziekan JM, Huon C, Berneburg I, Rahlfs S, Barale J-C, Feinstein SI, Fisher AB, Ménard D, Bozdech Z, Amino R, Touqui L, and Chitnis CE (2022). Human peroxiredoxin 6 is essential for malaria parasites and provides a host-based drug target. Cell Reports. 39(11): 110923. 10.1016/j.celrep.2022.110923
- Brancucci NMB, Gerdt JP, Wang C, De Niz M, Philip N, Adapa SR, Zhang M, Hitz E, Niederwieser I, Boltryk SD, Laffitte M-C, Clark MA, Grüring C, Ravel D, Blancke Soares A, Demas A, Bopp S, Rubio-Ruiz B, Conejo-Garcia A, Wirth DF, Gendaszewska-Darmach E, Duraisingh MT, Adams JH, Voss TS, Waters AP, Jiang RHY, Clardy J, and Marti M (2017). Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell. 171(7): 1532-1544.e15. 10.1016/j.cell.2017.10.020
- Asad M, Yamaryo-Botté Y, Hossain ME, Thakur V, Jain S, Datta G, Botté CY, and Mohmmed A (2021). An essential vesicular-trafficking phospholipase mediates neutral lipid synthesis and contributes to hemozoin formation in Plasmodium falciparum. BMC Biol. 19: 159. 10.1186/s12915-021-01042-z
- Sheokand PK, Narwal M, Thakur V, and Mohmmed A (2021). GlmS mediated knock-down of a phospholipase expedite alternate pathway to generate phosphocholine required for phosphatidylcholine synthesis in Plasmodium falciparum. Biochemical Journal. 478(18): 3429–3444. 10.1042/BCJ20200549
- Bhanot P, Schauer K, Coppens I, and Nussenzweig V (2005). A surface phospholipase is involved in the migration of plasmodium sporozoites through cells. J Biol Chem. 280(8): 6752–6760. 10.1074/jbc.M411465200
- Burda P-C, Roelli MA, Schaffner M, Khan SM, Janse CJ, and Heussler VT (2015). A Plasmodium Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane. PLoS Pathog. 11(3): e1004760. 10.1371/journal.ppat.1004760
- Ramaprasad A, Burda P-C, Koussis K, Thomas JA, Pietsch E, Calvani E, Howell SA, MacRae JI, Snijders AP, Gilberger T-W, and Blackman MJ (2023). A malaria parasite phospholipase facilitates efficient asexual blood stage egress. PLoS Pathog. 19(6): e1011449. 10.1371/journal.ppat.1011449
- Srivastava PN, and Mishra S (2022). Disrupting a Plasmodium berghei putative phospholipase impairs efficient egress of merosomes. Int J Parasitol. 52(8): 547–558. 10.1016/j.ijpara.2022.03.002
- Singh P, Alaganan A, More KR, Lorthiois A, Thiberge S, Gorgette O, Guillotte Blisnick M, Guglielmini J, Aguilera SS, Touqui L, Singh S, and Chitnis CE (2019). Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proc Natl Acad Sci USA. 116(35): 17498–17508. 10.1073/pnas.1900266116
- Flammersfeld A, Panyot A, Yamaryo-Botté Y, Aurass P, Przyborski JM, Flieger A, Botté C, and Pradel G (2020). A patatin-like phospholipase functions during gametocyte induction in the malaria parasite Plasmodium falciparum. Cellular Microbiology. 22(3): e13146. 10.1111/cmi.13146
- Dumaine JE, Tandel J, and Striepen B (2020). Cryptosporidium parvum. Trends in Parasitology. 36(5): 485–486. 10.1016/j.pt.2019.11.003
- Pollok RCG, McDonald V, Kelly P, and Farthing MJG (2003). The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol Res. 90(3): 181–186. 10.1007/s00436-003-0831-8
- Kennedy PGE (2019). Update on human African trypanosomiasis (sleeping sickness). J Neurol. 266(9): 2334–2337. 10.1007/s00415-019-09425-7
- Desquesnes M, Gonzatti M, Sazmand A, Thévenon S, Bossard G, Boulangé A, Gimonneau G, Truc P, Herder S, Ravel S, Sereno D, Jamonneau V, Jittapalapong S, Jacquiet P, Solano P, and Berthier D (2022). A review on the diagnosis of animal trypanosomoses. Parasit Vectors. 15: 64. 10.1186/s13071-022-05190-1
- Giordani F, Morrison LJ, Rowan TG, DE Koning HP, and Barrett MP (2016). The animal trypanosomiases and their chemotherapy: a review. Parasitology. 143(14): 1862–1889. 10.1017/S0031182016001268
- Hambrey PN, Mellors A, and Tizard IR (1981). The phospholipases of pathogenic and non-pathogenic Trypanosoma species. Mol Biochem Parasitol. 2(3–4): 177–186. 10.1016/0166-6851(81)90098-0
- Opperdoes FR, and van Roy J (1982). The phospholipases of Trypanosoma brucei bloodstream forms and cultured procyclics. Mol Biochem Parasitol. 5(5): 309–319. 10.1016/0166-6851(82)90038-x
- Hambrey PN, Tizard IR, and Mellors A (1980). Accumulation of phospholipase A1 in tissue fluid of rabbits infected with Trypanosoma brucei. Tropenmed Parasitol. 31(4): 439–443. 7233541
- Bowes AE, Samad AH, Jiang P, Weaver B, and Mellors A (1993). The acquisition of lysophosphatidylcholine by African trypanosomes. J Biol Chem. 268(19): 13885–13892. 8314756
- Masterson WJ, Raper J, Doering TL, Hart GW, and Englund PT (1990). Fatty acid remodeling: A novel reaction sequence in the biosynthesis of trypanosome glycosyl phosphatidylinositol membrane anchors. Cell. 62(1): 73–80. 10.1016/0092-8674(90)90241-6
- Sage L, Hambrey PN, Werchola GM, Mellors A, and Tizard IR (1981). Lysophospholipase 1 in Trypanosoma brucei. Tropenmed Parasitol. 32(4): 215–220. 7345684
- Richmond GS, and Smith TK (2007). A novel phospholipase from Trypanosoma brucei. Molecular Microbiology. 63(4): 1078–1095. 10.1111/j.1365-2958.2006.05582.x
- Richmond GS, and Smith TK (2007). The role and characterization of phospholipase A1 in mediating lysophosphatidylcholine synthesis in Trypanosoma brucei. Biochem J. 405(2): 319–329. 10.1042/BJ20070193
- Monic SG, Lamy A, Thonnus M, Bizarra-Rebelo T, Bringaud F, Smith TK, Figueiredo LM, and Rivière L (2022). A novel lipase with dual localisation in Trypanosoma brucei. Sci Rep. 12(1): 4766. 10.1038/s41598-022-08546-w
- Tounkara M, Boulangé A, Thonnus M, Bringaud F, Bélem AMG, Bengaly Z, Thévenon S, Berthier D, and Rivière L (2021). Novel protein candidates for serodiagnosis of African animal trypanosomosis: Evaluation of the diagnostic potential of lysophospholipase and glycerol kinase from Trypanosoma brucei. PLoS Negl Trop Dis. 15(12): e0009985. 10.1371/journal.pntd.0009985
- Eintracht J, Maathai R, Mellors A, and Ruben L (1998). Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J. 336 (Pt 3): 659–666. 10.1042/bj3360659
- Catisti R, Uyemura SA, Docampo R, and Vercesi AE (2000). Calcium mobilization by arachidonic acid in trypanosomatids. Mol Biochem Parasitol. 105(2): 261–271. 10.1016/s0166-6851(99)00186-3
- Das UN (2021). Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules. 11(12): 1873. 10.3390/biom11121873
- Ridgley EL, and Ruben L (2001). Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Mol Biochem Parasitol. 114(1): 29–40. 10.1016/s0166-6851(01)00234-1
- Ji Z, Nagar R, Duncan SM, Sampaio Guther ML, and Ferguson MAJ (2023). Identification of the glycosylphosphatidylinositol-specific phospholipase A2 (GPI-PLA2) that mediates GPI fatty acid remodeling in Trypanosoma brucei. J Biol Chem. 105016. 10.1016/j.jbc.2023.105016
- Tizard I, Nielsen KH, Seed JR, and Hall JE (1978). Biologically active products from African Trypanosomes. Microbiol Rev. 42(4): 664–681. 10.1128/mr.42.4.664-681.1978
- Tizard IR, and Holmes WL (1976). The generation of toxic activity fromTrypanosoma congolense. Experientia. 32(12): 1533–1534. 10.1007/BF01924436
- Tizard IR, Holmes WL, and Nielsen K (1978). Mechanisms of the anemia in trypanosomiasis: studies on the role of the hemolytic fatty acids derived from Trypanosoma congolense. Tropenmed Parasitol. 29(1): 108–114. 644654
- Tizard IR, Mellors A, Holmes WL, and Nielsen K (1978). The generation of phospholipase A and hemolytic fatty acids by autolysing suspensions of Trypanosoma congolense. Tropenmed Parasitol. 29(1): 127–133. 347651
- Nok AJ, Esievo KA, Ibrahim S, Ukoha AI, and Ikediobi CO (1993). Phospholipase A2 from Trypanosoma congolense: characterization and haematological properties. Cell Biochem Funct. 11(2): 125–130. 10.1002/cbf.290110208
- Rios LE, Vázquez-Chagoyán JC, Pacheco AO, Zago MP, and Garg NJ (2019). Immunity and vaccine development efforts against Trypanosoma cruzi. Acta Trop. 200: 105168. 10.1016/j.actatropica.2019.105168
- Florin-Christensen M, Florin-Christensen J, de Isola ED, Lammel E, Meinardi E, Brenner RR, and Rasmussen L (1997). Temperature acclimation of Trypanosoma cruzi epimastigote and metacyclic trypomastigote lipids. Molecular and Biochemical Parasitology. 88(1): 25–33. 10.1016/S0166-6851(97)00056-X
- Bertello LE, Alves MJ, Colli W, and de Lederkremer RM (2000). Evidence for phospholipases from Trypanosoma cruzi active on phosphatidylinositol and inositolphosphoceramide. Biochem J. 345 Pt 1: 77–84. 10.1042/0264-6021:3450077
- Salto ML, Bertello LE, Vieira M, Docampo R, Moreno SNJ, and de Lederkremer RM (2003). Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryot Cell. 2(4): 756–768. 10.1128/EC.2.4.756-768.2003
- Connelly MC, and Kierszenbaum F (1984). Modulation of macrophage interaction with Trypanosoma cruzi by phospholipase A2-sensitive components of the parasite membrane. Biochem Biophys Res Commun. 121(3): 931–939. 10.1016/0006-291x(84)90766-6
- Wainszelbaum M, Isola E, Wilkowsky S, Cannata JJ, Florin-Christensen J, and Florin-Christensen M (2001). Lysosomal phospholipase A1 in Trypanosoma cruzi: an enzyme with a possible role in the pathogenesis of Chagas’ disease. Biochem J. 355(Pt 3): 765–770. 10.1042/bj3550765
- Belaunzarán ML, Wainszelbaum MJ, Lammel EM, Gimenez G, Aloise MM, Florin-Christensen J, and Isola ELD (2007). Phospholipase A 1 from Trypanosoma cruzi infective stages generates lipid messengers that activate host cell protein kinase c. Parasitology. 134(4): 491–502. 10.1017/S0031182006001740
- Belaunzarán ML, Wilkowsky SE, Lammel EM, Bott E, Barbieri MA, and Durante de Isola EL (2013). Phospholipase A1: A novel virulence factor in Trypanosoma cruzi. Molecular and Biochemical Parasitology. 187(2): 77–86. 10.1016/j.molbiopara.2012.12.004
- Burza S, Croft SL, and Boelaert M (2018). Leishmaniasis. Lancet. 392(10151): 951–970. 10.1016/S0140-6736(18)31204-2
- Henriques C, Atella G, Bonilha V, and de Souza W (2003). Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitol Res. 89(2): 123–133. 10.1007/s00436-002-0728-y
- Chaves MM, Canetti C, and Coutinho-Silva R (2016). Crosstalk between purinergic receptors and lipid mediators in leishmaniasis. Parasit Vectors. 9(1): 489. 10.1186/s13071-016-1781-1
- Passero LFD, Laurenti MD, Tomokane TY, Corbett CEP, and Toyama MH (2008). The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol Res. 102(5): 1025–1033. 10.1007/s00436-007-0871-6
- Bordon MLAC, Laurenti MD, Ribeiro SP, Toyama MH, Toyama D de O, and Passero LFD (2018). Effect of phospholipase A2 inhibitors during infection caused by Leishmania (Leishmania) amazonensis. J Venom Anim Toxins Incl Trop Dis. 24: 21. 10.1186/s40409-018-0156-9
- Pawlowic MC, and Zhang K (2012). Leishmania parasites possess a platelet-activating factor acetylhydrolase important for virulence. Molecular and Biochemical Parasitology. 186(1): 11–20. 10.1016/j.molbiopara.2012.08.005
- Goswami A, Koley T, Rajan MV, Madhuri P, Upadhyay N, Das U, Kumar M, Ethayathulla AS, and Hariprasad G (2023). Structural Modelling of Platelet Activating Factor Acetyl Hydrolase in Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei: Implications on Therapeutics for Leishmaniasis, Chagas Disease, and Sleeping Sickness. Infect Drug Resist. 16: 2117–2128. 10.2147/IDR.S403411
- Bott E, López MG, Lammel EM, Carfagna IE, Durante de Isola EL, Ruybal P, Taboga O, Gimenez G, and Belaunzarán ML (2020). Cellular localization, cloning and expression of Leishmania braziliensis Phospholipase A1. Microb Pathog. 141: 104010. 10.1016/j.micpath.2020.104010
- Shakarian AM, McGugan GC, Joshi MB, Stromberg M, Bowers L, Ganim C, Barowski J, and Dwyer DM (2010). Identification, characterization, and expression of a unique secretory lipase from the human pathogen Leishmania donovani. Mol Cell Biochem. 341(1–2): 17–31. 10.1007/s11010-010-0433-6
- Gorman H, and Chadee K (2019). Entamoeba Histolytica: Biology and Host Immunity. In: Reference Module in Life Sciences. Elsevier; p B9780128096338907000. 10.1016/B978-0-12-809633-8.90748-9
- Ravdin JI, Murphy CF, Guerrant RL, and Long-Krug SA (1985). Effect of antagonists of calcium and phospholipase A on the cytopathogenicity of Entamoeba histolytica. J Infect Dis. 152(3): 542–549. 10.1093/infdis/152.3.542
- Long-Krug SA, Fischer KJ, Hysmith RM, and Ravdin JI (1985). Phospholipase A enzymes of Entamoeba histolytica: description and subcellular localization. J Infect Dis. 152(3): 536–541. 10.1093/infdis/152.3.536
- Said-Fernández S, and López-Revilla R (1982). Subcellular distribution and stability of the major hemolytic activity of Entamoeba histolytica trophozoites. Z Parasitenkd. 67(3): 249–254. 10.1007/BF00927659
- Said-Fernández S, and López-Revilla R (1983). Latency and heterogeneity of Entamoeba histolytica hemolysins. Z Parasitenkd. 69(4): 435–438. 10.1007/BF00927699
- Said-Fernández S, and López-Revilla R (1988). Free fatty acids released from phospholipids are the major heat-stable hemolytic factor of Entamoeba histolytica trophozoites. Infect Immun. 56(4): 874–879. 10.1128/iai.56.4.874-879.1988
- Vargas-Villarreal J, Martínez-Rodríguez H, Castro-Garza J, Mata-Cárdenas BD, González-Garza MT, and Said-Fernández S (1995). Identification of Entamoeba histolytica intracellular phospholipase A and lysophospholipase L1 activities. Parasitol Res. 81(4): 320–323. 10.1007/bf00931538
- González-Garza MT, Castro-Garza J, Cruz-Vega DE, Vargas-Villarreal J, Carranza-Rosales P, Mata-Cárdenas BD, Siller-Campos L, and Said-Fernández S (2000). Entamoeba histolytica: diminution of erythrophagocytosis, phospholipase A(2), and hemolytic activities is related to virulence impairment in long-term axenic cultures. Exp Parasitol. 96(2): 116–119. 10.1006/expr.2000.4554
- Loftus B et al. (2005). The genome of the protist parasite Entamoeba histolytica. Nature. 433(7028): 865–868. 10.1038/nature03291
- Anderson DM, Sato H, Dirck AT, Feix JB, and Frank DW (2015). Ubiquitin activates patatin-like phospholipases from multiple bacterial species. J Bacteriol. 197(3): 529–541. 10.1128/JB.02402-14
- Hysmith RM, and Franson RC (1982). Elevated levels of cellular and extracellular phospholipases from pathogenic Naegleria fowleri. Biochim Biophys Acta. 711(1): 26–32. 10.1016/0005-2760(82)90005-4
- Hysmith RM, and Franson RC (1982). Degradation of human myelin phospholipids by phospholipase-enriched culture media of pathogenic Naegleria fowleri. Biochim Biophys Acta. 712(3): 698–701. 10.1016/0005-2760(82)90300-9
- Fulford DE, and Marciano-Cabral F (1986). Cytolytic activity of Naegleria fowleri cell-free extract. J Protozool. 33(4): 498–502. 10.1111/j.1550-7408.1986.tb05649.x
- Barbour SE, and Marciano-Cabral F (2001). Naegleria fowleri amoebae express a membrane-associated calcium-independent phospholipase A(2). Biochim Biophys Acta. 1530(2–3): 123–133. 10.1016/s1388-1981(00)00069-x
- Cernikova L, Faso C, and Hehl AB (2018). Five facts about Giardia lamblia. PLoS Pathog. 14(9): e1007250. 10.1371/journal.ppat.1007250
- Vargas-Villarreal J, Escobedo-Guajardo BL, Mata-Cárdenas BD, Palacios-Corona R, Cortes-Gutiérrez E, Morales-Vallarta M, Sampayo-Reyes A, and Said-Fernández S (2007). ACTIVITY OF INTRACELLULAR PHOSPHOLIPASE A 1 AND A 2 IN GIARDIA LAMBLIA. Journal of Parasitology. 93(5): 979–984. 10.1645/GE-1038R3.1
- Mata-Cárdenas B, Hernández-García M, González-Salazar F, Garza-González J, Palacios-Corona R, Cortés-Gutiérrez E, and Vargas-Villarreal J (2012). Axenic cultivation and comparative phospholipase A2 activity of Giardia duodenalis in a serum-free medium. Acta Parasitologica. 57(3). 10.2478/s11686-012-0035-4
- Hahn J, Seeber F, Kolodziej H, Ignatius R, Laue M, Aebischer T, and Klotz C (2013). High Sensitivity of Giardia duodenalis to Tetrahydrolipstatin (Orlistat) In Vitro. PLoS One. 8(8): e71597. 10.1371/journal.pone.0071597
- Edwards T, Burke P, Smalley H, and Hobbs G (2014). Trichomonas vaginalis : Clinical relevance, pathogenicity and diagnosis. Critical Reviews in Microbiology. 1–12. 10.3109/1040841X.2014.958050
- McGregor JA, French JI, Jones W, Parker R, Patterson E, and Draper D (1992). Association of cervicovaginal infections with increased vaginal fluid phospholipase A2 activity. Am J Obstet Gynecol. 167(6): 1588–1594. 10.1016/0002-9378(92)91746-w
- Lubick KJ, and Burgess DE (2004). Purification and analysis of a phospholipase A2-like lytic factor of Trichomonas vaginalis. Infect Immun. 72(3): 1284–1290. 10.1128/iai.72.3.1284-1290.2004
- Vargas-Villarreal J, Mata-Cárdenas BD, Palacios-Corona R, González-Salazar F, Cortes-Gutierrez EI, Martínez-Rodríguez HG, and Said-Fernández S (2005). Trichomonas vaginalis: Identification of soluble and membrane-associated phospholipase A 1 and A 2 activities with direct and indirect hemolytic effects. Journal of Parasitology. 91(1): 5–11. 10.1645/GE-3338
- Escobedo-Guajardo B, González-Salazar F, Palacios-Corona R, Torres de la Cruz V, Morales-Vallarta M, Mata-Cárdenas B, Garza-González J, Rivera-Silva G, and Vargas-Villarreal J (2013). Trichomonas vaginalis acidic phospholipase A2: isolation and partial amino acid sequence. Acta Parasitologica. 58(4). 10.2478/s11686-013-0166-2
- Carlton JM et al. (2007). Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 315(5809): 207–212. 10.1126/science.1132894
- Pk S, M N, V T, and A M (2021). GlmS mediated knock-down of a phospholipase expedite alternate pathway to generate phosphocholine required for phosphatidylcholine synthesis in Plasmodium falciparum. The Biochemical journal. 478(18). 10.1042/BCJ20200549
–
ACKNOWLEDGMENTS
Costs were supported by Université de Bordeaux (https://www.u-bordeaux.fr), CNRS (https://www.cnrs.fr) and the Agence Nationale de la Recherche through the grants GLYCONOV (grant number ANR-15-CE-15-0025-01) and ADIPOTRYP (grant number ANR19-CE15-0004-01). This work was also funded by the Laboratoire d’Excellence (LabEx) “French Parasitology Alliance For Health Care” (ANR-11-LABX-0024-PARAFRAP, https://labex-parafrap.fr) and the "Fondation pour le Recherche Médicale" (FRM, https://www.frm.org/) ("Equipe FRM", grant n°EQU201903007845). The funders had no role in decision to publish or preparation of the manuscript.
COPYRIGHT
© 2023

Phospholipases A and Lysophospholipases in protozoan parasites by Hervé et al. is licensed under a Creative Commons Attribution 4.0 International License.









