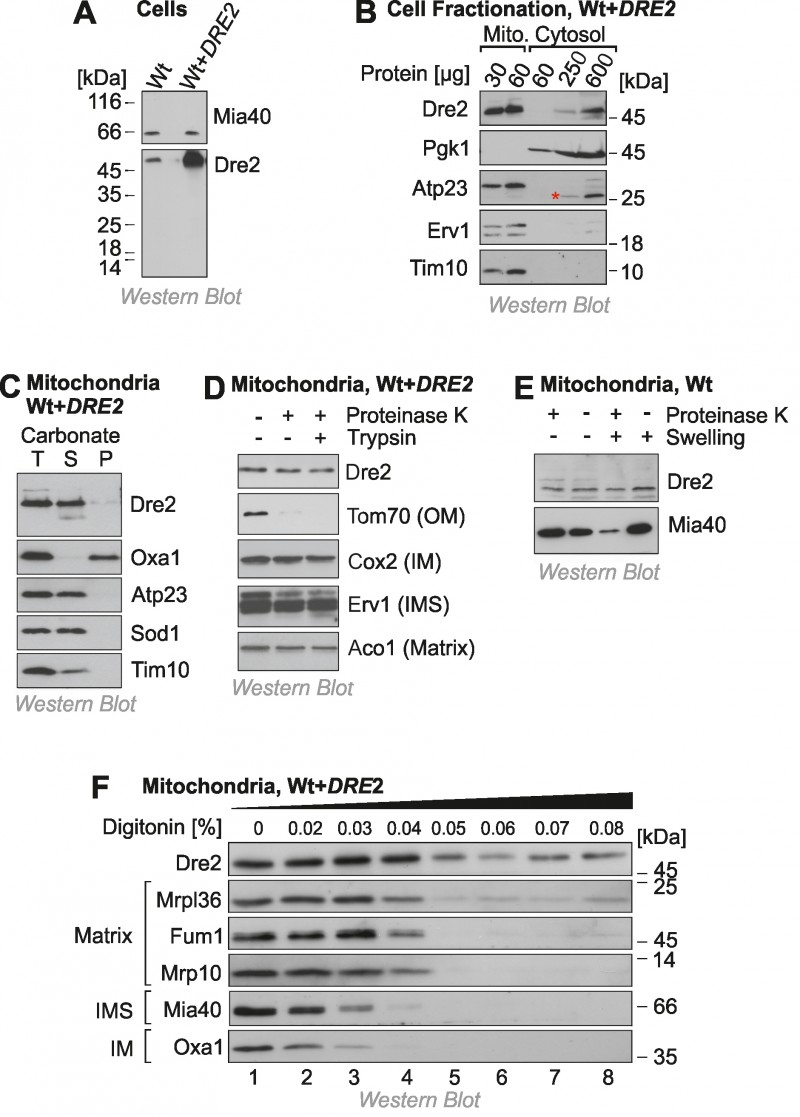
FIGURE 1: A fraction of Dre2 is associated with mitochondria.
(A) Wild type (Wt) and Dre2-overexpressing cells (0.5 ODs) were lysed and the levels of Dre2, and of Mia40 for control, were detected by Western blotting.
(B) Mitochondrial and cytosolic fractions were isolated from Dre2-overexpressing cells and analyzed by Western blotting. The asterisk indicates a crossreaction of the Atp23 antibody.
(C) Mitochondria (50 µg) isolated from Dre2-overexpressing cells were either directly loaded to the gel (T, total) or incubated with 0.1 M Na2CO3 for 30 min on ice before separation of soluble (S) and membrane (M) fractions by centrifugation for 30 min at 100,000 x g. The integral membrane protein Oxa1 and the soluble or membrane-associated proteins Atp23, Sod1, Tim10 were used as controls.
(D) Mitochondria were isolated from Dre2-overexpressing cells and incubated for 20 min with or without proteinase K or trypsin (100 µg/ml). The levels of Dre2 and control proteins were assessed by Western blotting. Only the outer membrane (OM) protein Tom70 was sensitive to the protease treatment. IM, inner membrane.
(E) Wild type mitochondria were incubated for 30 min with protease at isoosmotic or hypoosmotic (swelling) conditions. Whereas the IMS protein Mia40 was protease-accessible upon hypotonic rupturing of the outer membrane, Dre2 remained inaccessible.
(F) Mitochondria of Dre2-overexpressing cells were incubated with increasing concentrations of the detergent digitonin and exposed to proteinase K. Mitochondria were reisolated and the levels of the indicated proteins were detected by Western blotting.