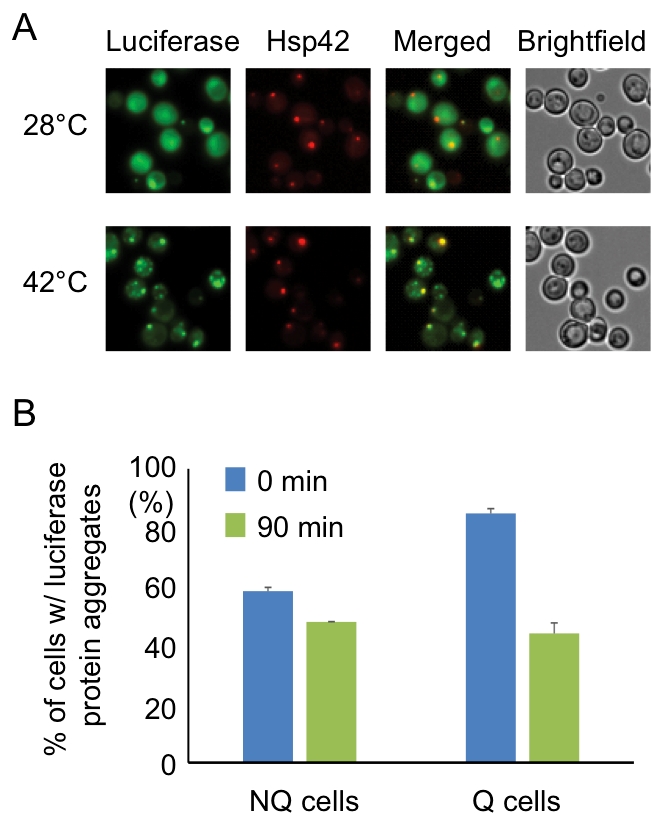
FIGURE 3: Hsp42-SPGs colocalize with heat shock-induced misfolded protein. (A) Heat shock-induced misfolded protein colocalizes to Hsp42-SPGs. Three-day stationary phase cells carrying Hsp42-BFP (Red) and an unstable mutant form of GFP-tagged luciferase (Green) were imaged before (28°C) and after (42°C) 30 min of mild heat shock at 42°C. The percentages of Hsp42-SPG-positive cells that colocalized with luciferase dots increased from 16 ± 2% to 70 ± 4% after heat shock. (B) Heat shock-induced protein aggregates are cleared more efficiently in Q cells. The luciferase-containing cells were monitored under a time-lapse microscope initiated before heat shock (see Materials and Methods). The numbers of cells containing heat shock-induced protein aggregates were counted immediately after heat shock (0 min) or after recovering at 28°C for 90 min (90 min). 48% of luciferase aggregates ([85 – 44]/85 = 48%) were cleared in Q cells, but only 17% ([58 – 48]/58 = 17%) were cleared in NQ cells. Three samples were analyzed in each condition and at least 100 cells were counted in each sample.
By continuing to use the site, you agree to the use of cookies. more information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this. Please refer to our "privacy statement" and our "terms of use" for further information.