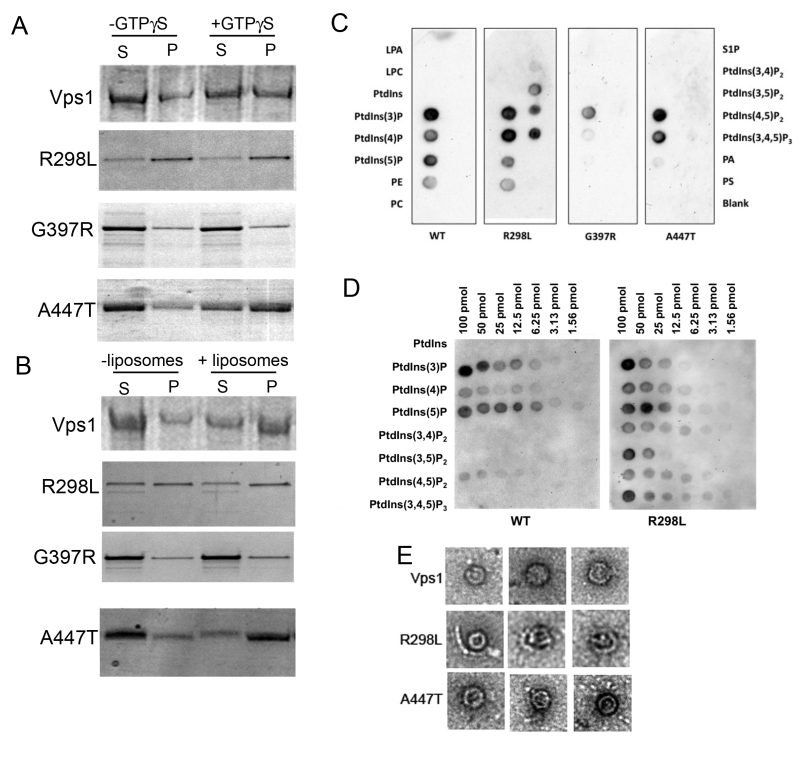
FIGURE 5: Biochemical analysis of Vps1 mutant proteins. His-tagged versions of the wild type and mutant Vps1 proteins were purified as described in the materials and methods. (A) Protein was incubated in the presence and absence of GTPγS which can lock the protein in the GTP bound form and stabilize the oligomeric form. Following centrifugation supernatant (S) and pellet (P) fractions were run on a gel and stained with Coomassie blue stain. (B) Protein was incubated in the presence and absence of liposomes. Samples were centrifuged to determine ability to interact with lipids and pellet with the liposomes. Following centrifugation supernatant (S) and pellet (P) fractions were run on a gel. Proteins were stained with Coomassie. (C) Binding to specific lipids was investigated using a PIP strip membrane approach. Proteins were incubated as described and binding assessed by western blotting. (D) A PIP array was used to further address the wider binding specificity of the R298L mutant compared to wild type Vps1. (E) Purified proteins were also analyzed following negative staining using electron microscopy. Representative oligomeric rings of Vps1 are shown.
By continuing to use the site, you agree to the use of cookies. more information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this. Please refer to our "privacy statement" and our "terms of use" for further information.