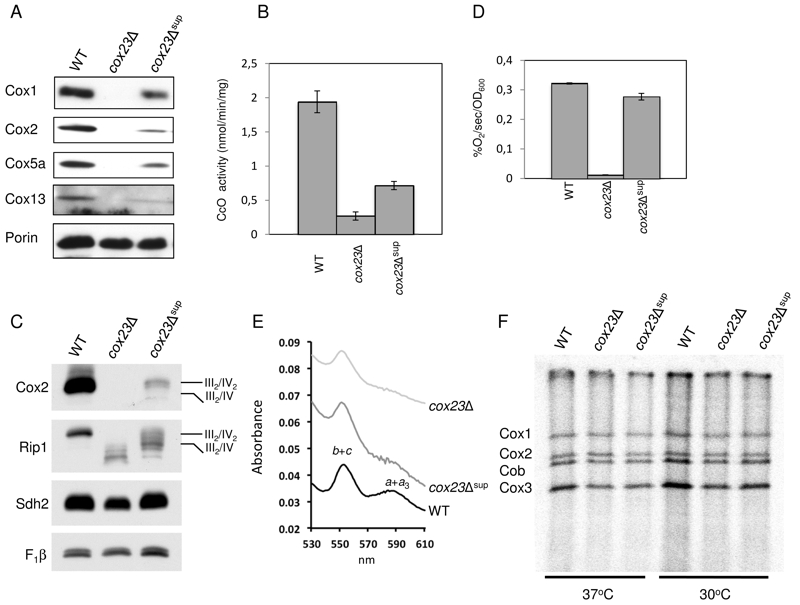
FIGURE 2: Functional characterization of cox23Δ suppressor.
(A) Steady-state concentration of representative Complex IV (CIV) subunits. Total mitochondrial protein (20 μg) were separated on 12% SDS-PAGE, transferred to nitrocellulose and probed with CIV subunit specific antibodies and porin as loading control.
(B) Mitochondria from WT, cox23Δ, and cox23Δ suppressor cells grown in liquid YPGal were purified and assayed for CcO activity (nmol cytochrome c oxidized/min/mg protein). The data represents the average of four independent experiments (error bars indicate standard deviation).
(C) Total mitochondrial protein (30 μg) were solubilized in 1% digitonin, the mitochondrial protein complexes separated on a blue-native gel, then transferred to PVDF. The complexes were visualized using antibodies specific to subunits of the respiratory complexes.
(D) WT, cox23Δ, and cox23Δ suppressor cells were grown in liquid YP-Galactose (YPGal) at 30°C overnight and carbon-swapped to YPGL for 10 hours before oxygen consumption was measured. The data represents the average of three independent experiments (error bars indicate standard deviation). 1000 cells were plated in YPD-agar to confirm viability (data not shown).
(E) Pyridine hemochromes analysis of mitochondrial hemes. Optical absorbance spectra were recorded of reduced minus oxidized cytochromes in the shown strains.
(F) Mitochondrial proteins of wild-type (WT), cox23Δ, and cox23Δ suppressor cells were pulse-labeled with [35S]-methionine for 7.5 min at 30°C and 37°C. Total protein were extracted and separated on 15% polyacrylamide gel, then dried and exposed to x-ray film.