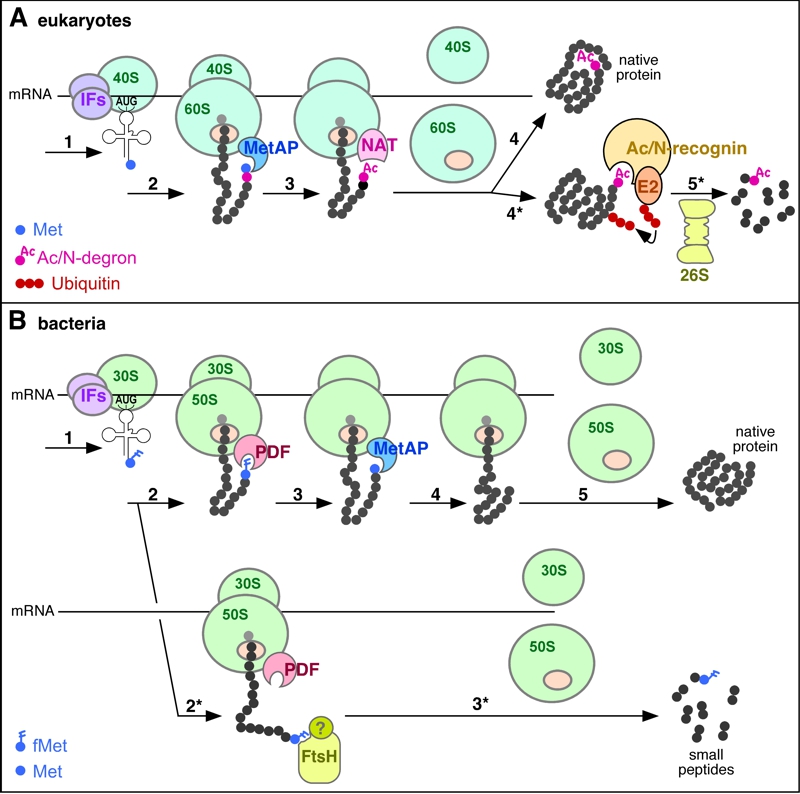
FIGURE 1: Role of N-terminal modifications in proteolytic targeting of nascent proteins.
(A) Role of Nt-acetylation in the degradation of nascent proteins in eukaryotes. Step 1, translation initiation by a complex of the small ribosomal subunit (40S), initiation factors (IFs) and Met-tRNA; step 2, translation elongation after binding of the large ribosomal subunit (60S); step 3: removal of Met by Met-aminopeptidase (MetAP); step 4: N-terminal acetylation of the α-amino group by Nt-acetyltransferase (NAT), followed by the completion of translation, and the release of ribosomal subunits as well as of the completed, folded protein, with sterically shielded Nt-acetyl group. Alternatively, in step 5*, an Ac/N-degron that is not repressed (owing, for example, to an impaired folding of nascent protein or the absence of a cognate binding partner) is bound by an Ac/N-recognin (a specific ubiquitin ligase) in complex with an E2 ubiquitin-conjugating enzyme. The resulting targeted protein is polyubiquitylated and processively degraded by the 26S proteasome.
(B) Possible role of N-terminal formyl-methionine (fMet) in the cotranslational quality control of nascent proteins in bacteria. Step 1, translation initiation by a complex of the small ribosomal subunit (30S), IFs and fMet-tRNA; step 2: translation elongation after binding of the large ribosomal subunit (50S); step 3, deformylation of fMet by the peptide deformylase (PDF) that transiently binds near the peptide exit tunnel of the ribosome; step 4: removal of deformylated Met by MetAP; step 5: completion of synthesis, followed by the release of ribosomal subunits and translated protein from mRNA. Alternatively, in step 2*, if the emerging nascent protein is not deformylated by PDF efficiently enough (for reasons mentioned in the main text) the fMet-bearing protein is recognized either directly by a protease (possibly by the FtsH protease) or by an adaptor (Ac/N-recognin) protein of unknown identity (indicated by a “?”), leading to processive degradation of the nascent protein in step 3*. Experimental data [1] suggest that the degradation via the fMet/N-end rule pathway occurs to a large extent cotranslationally as shown on the diagram.
1. Piatkov KI, Vu T, Hwang C-S, Varshavsky A (2015). Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. Microbial Cell. http://dx.doi.org/10.15698/mic2015.10.231