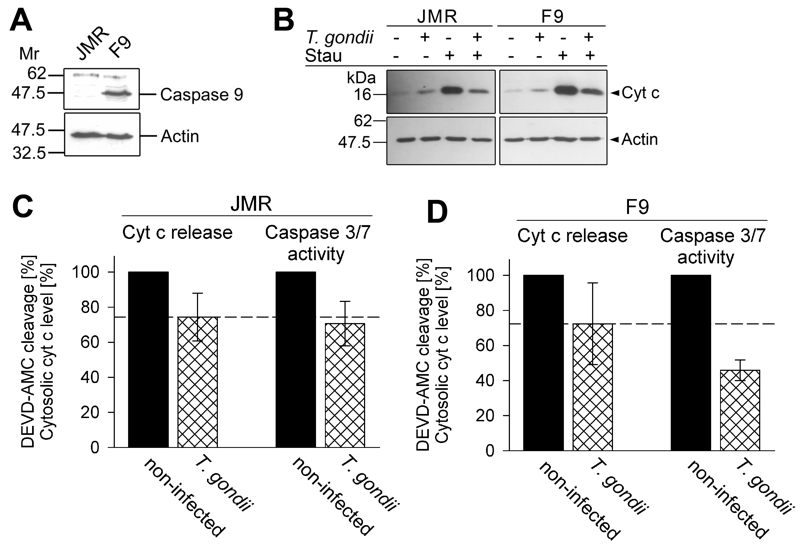
FIGURE 3: Total inhibition of the intrinsic apoptotic pathway by T. gondii considerably relies on a mechanism that operates down-stream of mitochondrial cytochrome c release and which relies on caspase 9.
(A) Total cell lysates were prepared from caspase 9-deficient Jurkat cells (clone JMR) and a reconstituted mutant thereof (F9) and were separated by SDS-PAGE. After protein transfer, nitrocellulose membranes were probed with antibodies recognizing caspase 9 and actin. Immune complexes were visualized using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
(B) JMR and F9 cells were infected with T. gondii for 24 hours (MOI 20:1) or were left non-infected and were then treated or not with staurosporine to trigger the cell-intrinsic PCD pathway. After 90 minutes, cytosolic proteins were isolated by digitonin lysis (digitonin-soluble extract). Proteins were resolved by SDS-PAGE and after transfer to nitrocellulose, were probed with antibodies recognizing cytochrome c (cyt c) or actin. Bound antibodies were visualized using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. Band intensities of cytosolic cytochrome c after treatment of cells with staurosporine were determined by densitometric analysis and were normalized to actin band intensities.
(C, D) Cells were infected with T. gondii and/or treated with staurosporine as described above (B). After cell lysis, cleavage of the caspase 3/7 substrate DEVD-AMC was measured fluorimetrically. Data represent the increase of cleaved substrate over time. For comparison, the levels of cytosolic cytochrome c as determined by densitometric analysis (B) are displayed. Results are expressed as mean percentages ± S.E.M. of at least three independent experiments; the caspase 3/7 activities and the levels of cytosolic cytochrome c in non-infected cells have been set to 100%.