Reviews:
Microbial Cell, Vol. 6, No. 4, pp. 184 - 196; doi: 10.15698/mic2019.04.673
Forty-five-year evolution of probiotic therapy
1 Departments of Microbiology & Immunology, and Surgery, Western University.
2 Lawson Health Research Institute.
Keywords: probiotics, Lactobacillus, urogenital, cardiovascular, gastrointestinal, therapy.
Abbreviations:
AAD – antibiotic associated diarrhea,
AD – atopic dermatitis,
BV – bacterial vaginosis,
GI – gastrointes-tinal,
HMP – Human Microbiome Project,
IBD – inflamma-tory bowel disease,
LGG – Lactoba-cillus rhamnosus GG,
NEC – necrotiz-ing enterocolitis,
RCT – random-ized controlled trial,
UTI – urinary tract infection.
Received originally: 22/07/2018 Received in revised form: 20/11/2018
Accepted: 23/11/2018
Published: 01/04/2019
Correspondence:
Dr. Gregor Reid, Lawson Health Research Institute, Room F3-106, PO BOX 5777, STN B London, ON N6A 4V2 Canada; Tel: +1 519-646-6100; gregor@uwo.ca
Conflict of interest statement: SPB has no conflicts of interests. GR is a consultant for Seed, a company producing probiotics.
Please cite this article as: Scarlett Puebla-Barragan and Gregor Reid (2019). Forty-five-year evolution of probiotic therapy. Microbial Cell 6(4): 184-196. doi: 10.15698/mic2019.04.673
Abstract
In the past forty-five years, the field of probiotics has grown from a handful of laboratory studies and clinical ideas into a legitimate research and translational entity conferring multiple benefits to humans around the world. This has been founded upon three principles: (i) the need for alternatives to drugs that either have sub-optimal efficacy or severe adverse effects; (ii) a growing interest in natural products and microbes, in particular catalyzed by studies showing the extent of microbes within humans and on our planet; and (iii) evidence on the genetics and metabolic properties of probiotic strains, and clinical studies showing their effectiveness. While some manufacturers have sadly taken advantage of the market growth to sell supplements and foods they term probiotic, without the necessary human study evidence, there are more and more companies basing their formulations on science. Adherence to the definition of what constitutes a probiotic, conclusions based on tested products not generalizations of the whole field, and applications emanating from microbiome research identifying new strains that provide benefits, will make the next forty-five years significantly changed approaches to health management. Exciting applications will emerge for cardiovascular, urogenital, respiratory, brain, digestive and skin health, detoxification, as well as usage across the world’s ecosystems.
INTRODUCTION
The emergence of a new field of science is exciting yet invariably faces challenges to its validity and acceptance of its scope of influence. This is certainly the case for probiotics, now defined as “Live microorganisms, that when administered in adequate amounts, confer a health benefit on the host” [1].
–
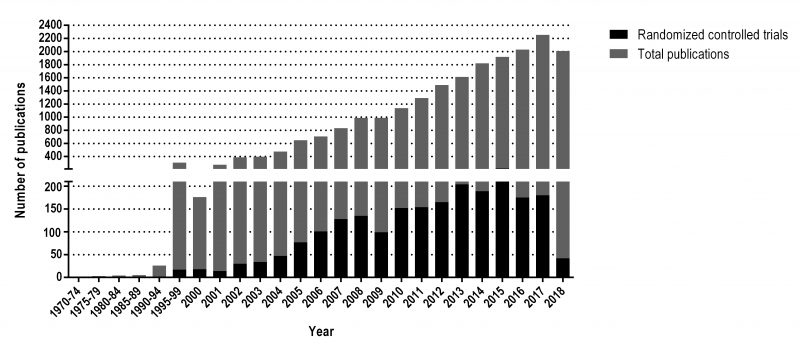
The information presented in the following review has been selected with the objective of examining some key elements of probiotics, which have played an important role in the rising of the number of publications on the topic from a handful to around 20,000 on PubMed since 1973, from which over 2,000 correspond to randomized controlled trials. A detail of the number of publications per year can be visualized in Figure 1.
–
THE MODERN-DAY ORIGINS OF PROBIOTICS
The first observations of beneficial bacteria were made by Elie Metchnikoff in 1905, when he proposed that the reason behind increased longevity in the Bulgarian population was due to the lactobacilli used to produce a yogurt commonly consumed in that region, and not the product itself as it was previously believed [2]. Nonetheless, although these remarks set the grounds for research on potential beneficial microorganisms, it was not until several decades later that formal research of probiotics begun.
–
Clinical observations by urologist Andrew Bruce in 1973, set the wheels in motion for considering lactobacilli as probiotics for the urogenital tract of women [3][4]. While the rest of the field was trying to develop vaccines and therapies against uropathogenic Escherichia coli, none of which have so far borne fruit, he believed that replenishment of lactobacilli into the vagina where E. coli were dominant after repeated urinary tract infections (UTI) and antibiotic treatments, might restore homeostasis and protect the host. He also wanted to apply the same idea to patients with ileal conduits, where establishment of a ‘normal’ microbiota appeared to reduce infection rates [5]. The latter never materialized but may be worth testing now with fecal microbiota transplant.
–
During the same era of 1960s-early 70s, Dwayne Savage and others were performing studies that showed the enormity and complexity of the intestinal microbiota in healthy subjects [3]. This was preceded by others reporting the diversity of microbes in the oral cavity [6].
–
The new interest in a limited microbial ecology discipline, seeded by the aforementioned studies, along with the introduction of the term ‘microbiome’ by Whipps et al. in 1988 [7][8], set the grounds from which the Human Microbiome Project (HMP) emerged.
–
Although before the early 2000’s most of the microbiology studies related to humans were interested in pathogenic organisms, reviews by David Relman and Stanley Falkow [9][10] stated the importance on paying attention to the endogenous microbes of the human body, as they could be determinant actors in health and disease. Furthermore, while the human genome project was being carried out, Julian Davies suggested that for it to be sufficiently relevant it was important to also understand the relationship between humans and the microorganisms that inhabit them [11]. The result was the HMP and Metahit Consortium that compiled an inventory of microbes in the mouth, gut, vagina, and skin of a group of humans [12][13].
–
In 2001 Joshua Lederberg described the ‘microbiome’ as “the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space and have been all but ignored as determinants of health and disease” [14]. Early interest on the microbiome led to an increased interest on performing a large-scale investigation on the human intestinal microbiome [12]. Consequently, in November 2005, an international meeting took place in Paris, were it was recommended that a Human Intestinal Metagenome Initiative (HIMI) should be started to better understand the role of the human intestinal microbiome [12]. Furthermore, with views of aiding in the accomplishment of the objectives of the HIMI, the formation of an International Metagenome Consortium was also recommended. This meeting initiated international collaboration to elucidate and better understand the relevance of the human microbiome.
–
In 2008, the HMP was funded as a NIH-sponsored initiative [12]. This project was created with a vision of using high-throughput technologies to characterise the human microbiome by analyzing samples from 300 healthy volunteers at 18 different body sites, as well to also understand the role of the microbiome in health and disease [15][16][17]. It was also expected that the knowledge obtained would provide a standardized data resource and new technology that would help to enhance the progression in this area of study, as well as to demonstrate the relevance of the understanding and manipulation of the microbiome as a tool to improve human health. The results from this project have been the isolation and sequencing of nearly 1,300 reference strains isolated from the human body [15][16].
–
The focus on beneficial microbes as distinct from pathogenic ones was all but ignored in the 1960s to early 2000s, and essentially deemed of interest only to microbial ecologists. Indeed, the President of the American Society of Microbiology even referred to probiotics as ‘snake oil’ sold from the back of covered wagons [18]. Such ignorance reflects on the person making the statement rather than the progress being made in the field, but it illustrates the challenges of acceptance. Criticism continues to this day, with researchers choosing to target probiotics under the illusion of them causing widespread harm and not being proven to be safe [19], when the evidence completely contradicts such views, and indeed probiotics are effectively used to offset drug side effects [20][21].
–
The updated definition of probiotics, introduced in 2001 [22] and reaffirmed in 2014 [1], along with the establishment of the International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2002 [23] were major factors in stimulating research in this area and emphasizing the importance of scientific rigour and production standards for probiotics. The growth of probiotic peer-reviewed publications to around 20,000 on the scientific search engine PubMed from just over 1,000 in 2002 reflects this impact (see Figure 1), this tool allows the user to search publications from several scientific life-sciences and medical databases. The breadth of the definition was intentional to allow capture of a range of host benefits. Subsequently, a range of terms have been used in the literature from psychobiotics, post-biotics, next-generation probiotics to baby-biotics, notably none adequately defined and none sufficiently different that they would fall outside the existing probiotic definition. These terms seem to group probiotics in very specific clusters, with very definite uses, when in reality, most of the probiotic strains available will have more than one targeted benefit on the host. Therefore, such terminology is confusing to healthcare providers, producers and consumers. If someone truly wants to develop new terminology, they need to define the term and its scope, and show how it should be interpreted, then explore with experts in the field whether it is applicable and is likely to be accepted by the wider community.
THE PRINCIPLES OF PROBIOTIC THERAPY
While credit is given to Elie Metchnikoff [2] for aligning fermented foods with longevity, and therefore stimulating the idea of developing such foods, the modern-day probiotics were designed for two basic reasons.
–
Firstly, probiotics are used to replenish organisms that are naturally in a given niche but whose numbers have been depleted and illness has occurred. This could be the use of Lactobacillus crispatus in the vagina to counter bacterial vaginosis or ascension of E. coli into the bladder [24].
–
Secondly, probiotic strains are selected because they have properties that counter pathogens/conditions causing illness, with an aim of restoring health and ideally allowing the indigenous beneficial microbes to return. The example would be to orally administer Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14, which are not species highly prevalent in the vagina, but whose administration results in recovery from infection and the return of indigenous L. crispatus and L. iners [25].
–
These approaches essentially manipulate the existing microbiome, even though the term ‘microbiome’ was not yet created when the probiotic applications were first conceived. Critics have argued that probiotics do not alter the gut microbiome, leading to some people then regarding them as a waste of money [26]. However, this shows a lack of understanding of the field on several counts. The 16S rRNA methods used to determine alterations in microbial abundance in the gut are far from ideal, and certainly not sensitive enough to verify that no changes are induced by probiotic intake. Furthermore, it is not a prerequisite for a probiotic to confer benefits by having to significantly change the host’s gut microbiota. Rather, the health benefit can be accrued through metabolites produced by the probiotic strains as they pass through the intestine [27], and by interactions with the host’s own metabolism [28] and immune system even in healthy adults [29].
WHERE HAVE THE EARLY STUDIES TAKEN US?
By 1997, nearly a hundred studies had been carried out on probiotics for the treatment of infections to warrant a literature review. Although the authors used the term pharmaceutical probiotics, this simply reflected their use to treat disease and the antiquated categorization of only drugs being able to make that claim. They concluded that probiotics could be used to treat and prevent infectious diseases [30]. Twenty years later and this application of probiotics against infectious diseases has expanded [31], and yet the use of these products for this purpose is sporadic at best. This is in large part to the reticence of medical practitioners accepting and implementing health interventions, especially those not regulated as drugs [32]. This seventeen-year gap seems hard to accept, especially when respected groups of peers have advocated the use of probiotics, particularly for digestive function and disease [33][34][35].
–
Undoubtedly, the most extensively researched application for probiotics is to promote gastrointestinal (GI) health. Although it has been commonly believed that the GI tract is sterile in utero, and therefore that its colonization does not occur until birth [36], recent studies have found that the placenta, amniotic fluid, and the umbilical cord harbour microorganisms [17][37][38][39][40]. These findings, as well as the fact that the meconium (first infant stool) is not sterile, provide a rationale to believe that the human GI tract begins its colonization during fetal development [17][41][42]. However, the presence of a fetal microbiota has been fiercely contested [43][44]. Nonetheless, most of the GI colonization happens postpartum [45]. As humans develop, so does their gut microbiome, which is influenced by factors such as age, diet, stress, geography, and drug intake [17]. The human gut is a complex ecosystem with a dynamic interaction between microorganisms, nutrients, and the host [46]. Therefore, probiotic supplementation, to target and improve the gut microbiome, has been extensively researched and found to be helpful in several GI conditions.
–
An example is the use of probiotics to improve gut function and maturity of neonates [17]. For instance, a systematic review of 25 randomized controlled trials (RCT; n = 4527) where probiotics were administered to preterm (gestation <37 weeks) or low-birth-weight (<2500 g) neonates, showed benefits of probiotic supplementation such as shorter full enteral feeds, improved feed tolerance, better weight gain and growth velocity, and decreased transition time from orogastric to breast feeds; no adverse effects where reported [47]. Nonetheless, more evidence is still needed to support the claim that probiotic supplementation at an early age is safe and beneficial [48].
–
Furthermore, one of the most thoroughly examined areas for the use of probiotics, is the prevention of antibiotic-associated diarrhea (AAD), nonetheless it is important to note that not all probiotic strains will be as effective as others for this purpose. For instance, a recent systematic review compared the efficacy and tolerability of different probiotics for AAD, authors examined 51 RCTs (n = 9,569) and found that management with Lactobacillus rhamnosus GG (LGG) was significantly superior that with any other strain of probiotics when used for preventing the condition. While in terms of reducing Clostridium difficile infection rate, Lactobacillus casei had higher efficacy [49]. Therefore, the type of probiotic to be used should always depend on the situation of the patient, as well as the desired outcome.
–
Another GI condition where probiotics have been proven to be helpful is Helicobacter pylori colonization, which is a problem for approximately 50% of the world population [50]. There is evidence that they can be helpful in the eradication of H. pylori by inhibiting its growth. A meta-analysis of 14 RCTs (n = 1,671) found that there is a substantial 83.6% eradication rate of the pathogen with probiotic treatment vs. 74.9% with conventional therapy [33]. Additionally, probiotics significantly reduced the side effects of conventional therapy when used as a conjoint treatment. In terms of recommended strains for this condition, Lactobacillus reuteri DSM 17938 [51][52] and LGG [53] have been found to be effective in combination with conventional therapy.
–
Remarkably, the Working Group on Probiotics of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition, with the objective of providing evidence-based recommendations, published a document that reviewed existent RCTs and systematic reviews on the use of probiotics for the prevention of AAD in children [33]. Based on their results, they strongly recommend the use of either LGG or Saccharomyces boulardii for the prevention of the condition in children. Meanwhile, in terms of preventingClostridium difficile-associated diarrhea, the Working Group recommends the use of S. boulardii, although with the caveat that the quality of evidence is still limited and requires further investigation. Interestingly, these recommendations are in line with those proposed for children of the Asia-Pacific region were, in addition to LGG and S. boulardii, L. reuteri was also suggested for the management of infantile colic and as a conjoint treatment with other probiotics for the management of H. pylori [34].
–
Inflammatory bowel disease (IBD) is a chronic condition of the large intestine that includes ulcerative colitis (UC) and Crohn’s disease (CD). Several studies have investigated the potential benefits of administration of probiotics to patients with IBD. A meta-analysis that reviewed 22 RCTs that recruited adults with either UC or CD, with the objective of comparing probiotics to the standard treatment of 5-aminosalicylates (5-ASAs), and placebo [54] The probiotic VSL#3 was stated to be effective in inducing remission in active UC. However, for management of CD, the benefits and efficacy of probiotics are not well established, and further research is required before they could be recommended for primary care.
–
Not all studies of probiotics have end points for GI conditions. One highly cited example is a study published in 2001 by Finnish researchers on atopic dermatitis (AD) [55], a chronic inflammatory disease of the skin which has become a major public-health problem [56]. It is estimated to affect 15-20% of children and 1-3% of adults worldwide, and its prevalence has increased by 2-3 fold recently (particularly in low-income and industrialized countries) [57][58].
–
The immune mechanisms associated with AD are characterized by a biphasic inflammation, meaning that at an initial and acute phase there is a predominant Th2-biased immune response, while chronic lesions are characterised by a Th1/Th dominance [58][59]. Regulatory T cells as well as the innate immune system are altered in the skin of patients with AD [60]. The link between childhood impaired immune system development and an altered intestinal microbiome increasing the susceptibility to allergic and autoimmune disease [61], led to a study by Majamaa and Isolauri which found that probiotic LGG promoted local-antigen specific immune responses that prevented gut permeability defects [62]. The researchers then hypothesized that oral administration of probiotics could be helpful in the treatment of food allergies by diminishing intestinal inflammation. Levels of α1-antitrypsin decreased significantly as did the concentration of fecal tumor necrosis factor-α [63]. It was these studies that formed the basis for the randomized placebo-controlled trial in which LGG was administered prenatally to mothers with at least one first-degree relative (or partner) with atopic eczema, allergic rhinitis, or asthma, and to their infants for 6 months after birth. Their findings showed a significant decrease in the frequency of AD in the group that consumed probiotics vs the placebo [55]. The researchers followed the children for a further four years and reported there was still a reduced risk of AD [64]. Although this study stimulated much interest in the use of probiotics in infants and pregnant women, as well as to aid in the modulation of immune diseases, subsequent studies have varied in their findings [65][66], and the totality of evidence has been deemed insufficient to support a therapeutic claim [67]. In terms of using probiotics to prevent AD, a meta-analysis of fourteen studies demonstrated a moderate decreased incidence (RR = 0.79 [95% CI = 0.71-0.88]) [68].
–
Some probiotic strains appear to confer benefits as adjuvant therapy for the treatment of adults [69]. However, a recent meta-analysis of thirteen studies aimed at using probiotic strains to treat children with AD, concluded that the evidence was not sufficiently robust for the category in general [70].
–
Another major area of interest in probiotic research is their use to manage urogenital tract conditions, mostly because of the high prevalence of UTI and bacterial vaginosis (BV), and the lack of effective treatment options [71][72]. A number of probiotic strains have been developed to prevent urogenital infections, Lactobacillus acidophilus A-212, Lactobacillus rhamnosus A-119, with Streptococcus thermophilus A-336; Lactobacillus rhamnosus PBO1 with Lactobacillus gasseri EN-153471 (EB01); and Lactobacillus rhamnosus Lcr35 in vaginal ovules. Strains L. rhamnosus GR-1 and L. reuteri RC-14 are the only ones approved for oral use in Canada and the United States. The positive early clinical studies performed with GR-1 and RC-14 showing improved vaginal microbiota and reduced infection recurrence, as reviewed elsewhere [73] have been confirmed by others [74][75][76]. The mechanisms of action include an increased ascension of probiotic and indigenous lactobacilli from rectal skin to the vagina, reduced pathogens ascension, plus localized inhibition and displacement of pathogens and priming of antimicrobial defenses [77][78]. Anti-fungal effects have also been reported [79][80], coinciding with improved curing of vulvovaginal candidiasis [81].
–
The vaginal administration of probiotic Lactobacillus using suppositories pioneered in the late 1980s [4], has since led to other strains being tested for urogenital health including Lactobacillus crispatus CTV05 to prevent recurrence of UTI [24], Lactobacillus rhamnosus IMC 501 with Lactobacillus paracasei IMC 502 [82] for vaginal health, Lactobacillus rhamnosus Lcr35 to aid in the management of BV and vulvovaginal candidiasis [83][84], and Lactobacillus gasseri EN-153471 (EB01) to help treat BV along with antibiotics [85].
–
A recent study of the genome of L. rhamnosus GR-1 showed why it is better adapted to the vagina than Lc35 and LGG strains, by having a unique cluster for exopolysaccharide production, metabolize lactose and maltose, and better withstand oxidative stress [86].
–
Table 1 includes a summary of the strains with significant clinical evidence for their use as probiotics, as well as the conditions for which they have been studied.
–
TABLE 1. Probiotic strains and the condition they target. |
 |
| [51][52][53][54][55][84][85][101][122][123][124][125][126][127][128][129][130][131][132][133][134] |
THE ULTIMATE REBUKE OF THE SNAKE OIL MYTH
Although the level of scientific and clinical evidence for probiotics should have by now rebuked the statements from even presidents of microbiology societies [18], and the 17-year change timeframe has now passed [32], the ability to save lives should surely be the ultimate proof of validity. A recent large study from India showed such an ability in preventing sepsis in newborns [87], and the ability of numerous probiotics to prevent necrotizing enterocolitis (NEC) in low birth weight, premature babies is extremely convincing. In fact, a meta-analysis of 29 RCTs (n = 2,310) that looked at the effect of probiotics on experimental NEC in animal models, reached the conclusion that probiotics help to significantly reduce NEC by different mechanisms which include modulating immune function as well as by regulating inflammation, tissue injury, gut barrier and intestinal dysbiosis [88]. Furthermore, a recent meta-analysis evaluated 37 RCTs (n = 5,033) that looked at the effect of probiotic administration to infants of less than 37 weeks of gestation or who were born weighing less than 2.5 kg, results showed that probiotics are indeed beneficial in the prevention of severe NEC and death in preterm infants [89]. Yet, despite the evidence, and highly successful implementation in neonatal intensive care units in Canada, Australia and elsewhere, American hospitals appear reluctant to embed it into practice.
–
The neonatal intensive care unit is essentially a box in which the environment is mostly filled by pathogenic, drug resistant pathogens apart from the indigenous microbiota of the attendants. It is no surprise that babies requiring intubation, intravenous fluids and intensive care, and invariably given antibiotics which destroy beneficial as well as pathogenic bacteria, are at high risk of NEC. The only means of administering to them beneficial bacteria is through mother’s milk, and that is dependent on whether the mother produces milk and feeds it to the baby. The immature gut and immune system place the newborn at high risk of NEC, so it is perhaps not surprising that the administration of probiotic bacteria protects against the pathogens and improves gut barrier function. The healthcare savings from preventing NEC are enormous, so it seems counter intuitive to not make probiotic therapy standard practice.
–
However, many clinicians are still reluctant to prescribe probiotics, perhaps due to fear and lack of knowledge about the area, which is further heightened by isolated reports of probiotic-induced sepsis [90]. Nonetheless, these cases are rare and often occur in immunocompromised patients. Also, the evidence available shows that, in most patients, the benefits outweigh the risks. In fact, a meta-analysis from 2016, found that there is a decreased incidence of culture-proven sepsis when probiotics are administered [91]. But, it is understandable, that practitioners proceed with caution when considering the administration of probiotics to high risk patients, and a guide to probiotics for this purpose has been published [20].
–
Despite guidelines for what constitutes a probiotic being published in 2002 [22], meta-analyses often fail to appreciate that there are strain to strain differences [92], and pool together studies performed on multiple product formulations. If a product has not been proven to benefit humans, it should not be called probiotic and therefore not included in a meta-analysis. If a probiotic for one condition fails in another, then it should not be recommended for the latter, and physicians should not then conclude that all probiotics work or don’t work. In some ways it is like comparing Warfarin with Lipitor, when both drugs have different purposes, even if both might benefit heart health. If different probiotics provide benefits for the same condition, as it has been found for NEC [88][89], then it seems reasonable to include them in a meta-analysis. However, a lack of understanding of probiotics can lead to scientific publications that make unsubstantiated conclusions and find their way to the mainstream media claiming that probiotics might not be as useful as commonly believed [93] or deeming them even potentially harmful to the host microbiota [94]. To make things even worse, when reporting these studies to the lay audience, the BBC went as far as to use a sensationalist headline, labeling probiotics as ‘quite useless’, without considering that the experimental design of said studies did not provide sufficient evidence to make that sort of generalizations or any clinical claims [95]. This is like stating all drugs are quite useless. Sadly, even though renowned experts in the field [96][97], and even the International Scientific Association for Probiotics and Prebiotics [98], made statements that pointed out the many flaws of this argument, the idea had already spread through mainstream media and the damage will be hard to revert.
THE NEXT FORTY-FIVE YEARS
Predicting the future has never been easy and is usually based on what we know today. To that extent, it seems clear that probiotics will be used to reduce depression and anxiety [99][100], and potentially other forms of mental illness. Certainly, trials are underway using fecal microbiota transplant for multiple sclerosis, Parkinson’s disease, and dementia, so within forty-five years, the strains able to confer an effect will have been identified, tested and be part of a microbial intervention medical practice. A more profound step will be to implant probiotic strains directly into the brain, perhaps to counter disease-causing organisms, or to produce certain chemicals, such as γ-aminobutyric acid or serotonin at specific sites to try and improve function. This will require an ability to manipulate the strains and control their spread beyond the implanted site – all well within the capabilities of molecular genetics and biomedical engineering. It is unlikely that bacteriophages or organisms like Bacillus thuringiensis var. israelensis that kill mosquito larvae, will be defined as probiotics given that they don’t benefit their host bacterium, but they too could be candidates for implantation into the brain, as might microbes that kill viruses. The ethical issues may take longer to unravel, depending on what metabolites are produced, and what functions they influence.
–
There will be no harder ethical questions raised than if probiotics influence organ development of the fetus and its life-long course. This is certainly feasible given that AD risk can already be reduced with maternal treatment. Whether the probiotics will be implanted into the uterus or their functions conveyed via the mother’s metabolism remains to be seen, but either could occur. There are clearly compounds, including neurochemicals, vitamins, lipids and peptides produced by microbes that already influence organ development and function [101][102][103][104][105]. There is also preliminary evidence for improving cognitive function in adults [106].
–
The research necessary to provide accurate modulation of fetal determinants of health will require an understanding of the extent to which microbes and their metabolites, whether in the mother or uterine/placental environment, influence human physiology without inducing long term adversity. This will be difficult to achieve and will likely require sensory systems that detect changes at the sub-cellular level. Probes like the iKnife [107] could potentially be minimized further, or nano-detection systems put in place [108]. Determining the contribution of nutrients, hormones and stem cell activities will further complicate the identification of critical microbes to be administered to optimize fetal development. But studies in which probiotic strains are already being used during pregnancy will allow assessments of the newborn’s organs and early year development, that will at least allow hypothesis generation, and markers to be available for testing different interventions, such as high folate producing probiotics.
–
Two other areas that will see significant advances in the next forty-five years are probiotics for cardiovascular management and to reduce uptake and damage caused by environmental toxins. There is already a growing literature on probiotic strains lowering cholesterol and improving blood pressure [109][110], even meta-analyses have found a significant reduction of serum total cholesterol when administering certain probiotics (namely L. acidophilus + B. lactis, VSL#3, and L. plantarum) [101]. Once comparative studies against statins are performed, we will know the percentage risk reduction for cardiovascular disease and be able to identify patients who can benefit from the probiotics prior to needing the drugs or as an adjunct to the statins. The animal studies showing a further effect on cardiac remodeling to reduce heart failure after ischemic injury [111][112], likely through increasing adiponectin, warrants human studies to determine if probiotic strains given right after infarction might increase longevity. Another application could be for Bifidobacterium strains to lower p-cresol sulfate, trimethylamine n-oxide and indole levels which are known risk factors for atherosclerosis [113][114].
–
Lastly, in a world with ever-increasing environmental pollution [115], the question is not when water will become pure enough to drink, as that day will never come, but how can humans cope with the inevitable intake of toxic products? The oro-gastrointestinal microbes provide a natural barrier that could be further enhanced by probiotics. It is well known that bacteria in the intestine can increase or decrease drug toxicity and uptake [116], and examples are mounting of probiotic bacteria reducing uptake of inadvertent intake of toxic compounds [117][118][119]. Blocking uptake through binding to the compounds or degrading them while they are in the GI tract, will become an application of probiotics, made even more effective by matching strains with foods and water whose toxin content is known through detectors that will become widely available. The ability of consumers to extract toxins and the many pharmaceutical agents contaminating our water supply [120] at their tap, will become standard in households, but ingesting foods with microbes that are highly adapted to detoxification will further help to reduce the health effects of these chemicals.
IN SUMMARY
The past forty-five years have seen probiotic microbes identified, tested, and applied to patients and consumers around the world. The over 40 billion-dollar market [121] reflects not only an interest in natural therapies and desire to avoid drugs that are often ineffective or with severe side-effects, but it has come about through rigorous scientific research. There may never be sufficient large, randomized placebo-controlled trials to satisfy all critics, but the numbers of lives saved and enhanced by probiotics continue to grow. As technology advances, the future will see different species applied in novel ways to further improve human well-being, and indeed the health of a range of other hosts from fish to honey bees, livestock and wildlife.
REFERENCES
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, and Sanders ME (2014). The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8): 506–514. doi: 10.1038/nrgastro.2014.66
- Metchnikoff E (1908). The prolongation of life; optimistic studies. G.P. Putnam’s Sons, New York and London.
- Bruce AW, Chadwick P, Hassan A, and VanCott GF (1973). Recurrent urethritis in women. Can Med Assoc J 108(8): 973–6. PMID: 4633489.
- Bruce AW, and Reid G (1988). Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol 34(3): 339–43. doi: 10.1139/m88-062
- Dubos RJ, Savage DC, and Schaedler RW (1967). The indigenous flora of the gastrointestinal tract. Dis Colon Rectum 10(1): 23–34. doi: 10.1007/BF02617382
- Socransky SS, Gibbons RJ, Dale AC, Bortnick L, Rosenthal E, and Macdonald JB (1963). The microbiota of the gingival crevice area of man—I. Arch Oral Biol 8(3): 275–280. doi: 10.1016/0003-9969(63)90019-0
- Whipps M, Lewis K, and Cooke RC (1988). Mycoparasitism and plant disease control. In: Burge M, editor Fungi Biol. Control Syst. Manchester University Press, New York; pp 161–87.
- Prescott SL (2017). History of medicine: Origin of the term microbiome and why it matters. Hum Microbiome J 4: 24–25. doi: 10.1016/j.humic.2017.05.004
- Relman DA (2002). New technologies, human-microbe interactions, and the search for previously unrecognized pathogens. J Infect Dis 186(s2): S254–S258. doi: 10.1086/344935
- Relman D, and Falkow S (2001). The meaning and impact of the human genome sequence for microbiology. Trends Microbiol 9(5): 206–208. doi: 10.1016/S0966-842X(01)02041-8
- Davies J (2001). In a map for human life, count the microbes, too. Science 291(5512): 2316b–2316. doi: 10.1126/science.291.5512.2316b
- Peterson J et al. (2009). The NIH Human Microbiome Project. Genome Res 19(12): 2317–2323. doi: 10.1101/gr.096651.109
- Qin J et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285): 59–65. doi: 10.1038/nature08821
- Lederberg J, and McCray AT (2001). ‘Ome sweet ‘omics—A genealogical treasury of words. Sci. 15(7): 8.
- The Human Microbiome Project Consortium (2012). A framework for human microbiome research. Nature 486(7402): 215–221. doi: 10.1038/nature11209
- The Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486(7402): 207–214. doi: 10.1038/nature11234
- Cresci GA, and Bawden E (2015). The gut microbiome: what we do and don’t know. Nutr Clin Pract 30(6): 734–746. doi: 10.1177/0884533615609899
- Atlas RM (1999). Probiotics – snake oil for the new millennium? Environ Microbiol 1(5): 377–382. doi: 10.1046/j.1462-2920.1999.00063.x
- Bafeta A, Koh M, Riveros C, and Ravaud P (2018). Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota. Ann Intern Med 169(4): 240. doi: 10.7326/M18-0343
- Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, and Leyer G (2016). Probiotic use in at-risk populations. J Am Pharm Assoc 56(6): 680–686. doi: 10.1016/j.japh.2016.07.001
- Feng J-R, Wang F, Qiu X, McFarland L V, Chen P-F, Zhou R, Liu J, Zhao Q, and Li J (2017). Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol 73(10): 1199–1208. doi: 10.1007/s00228-017-2291-6
- FAO/WHO Working Group (2002). Report of a joint FAO/WHO expert consultation on evaluation of health and nutrition properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina. Available at: www.fao.org/3/a-a0512e.pdf [Accessed 20.11.2018].
- Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, Roberfroid M, Rowland I, Cherbut C, and Klaenhammer TR (2003). New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37(2): 105–18. PMID: 12869879.
- Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, and Stamm WE (2011). Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent Urinary Tract Infection. Clin Infect Dis 52(10): 1212–1217. doi: 10.1093/cid/cir183
- Macklaim JM, Clemente JC, Knight R, Gloor GB, and Reid G (2015). Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb Ecol Heal Dis 26: 27799. doi: 10.3402/mehd.v26.27799
- Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, and Pedersen O (2016). Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 8(1): 52. doi: 10.1186/s13073-016-0300-5
- McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, Chervaux C, Knights D, Lozupone CA, Knight R, Duncan AE, Bain JR, Muehlbauer MJ, Newgard CB, Heath AC, and Gordon JI (2011). The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 3(106): 106ra106-106ra106. doi: 10.1126/scitranslmed.3002701
- Bazanella M, Maier T V, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, Autran C, Walter J, Bode L, Schmitt-Kopplin P, and Haller D (2017). Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr 106(5): 1274–1286. doi: 10.3945/ajcn.117.157529
- Harbige LS, Pinto E, Allgrove J, and Thomas L V. (2016). Immune response of healthy adults to the ingested probiotic Lactobacillus casei Shirota. Scand J Immunol 84(6): 353–364. doi: 10.1111/sji.12495
- McFarland L V, and Elmer GW (1997). Pharmaceutical probiotics for the treatment of anaerobic and other infections. Anaerobe 3(2–3): 73–78. doi: 10.1006/anae.1996.0062
- Reid G (2017). Probiotic use in an infectious disease setting. Expert Rev Anti Infect Ther 15(5): 449–455. doi: 10.1080/14787210.2017.1300061
- Morris ZS, Wooding S, and Grant J (2011). The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 104(12): 510–520. doi: 10.1258/jrsm.2011.110180
- Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S, Orel R, Shamir R, Vandenplas Y, van Goudoever JB, and Weizman Z (2016). Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr 62(3): 495–506. doi: 10.1097/MPG.0000000000001081
- Cameron D, Hock QS, Kadim M, Mohan N, Ryoo E, Sandhu B, Yamashiro Y, Jie C, Hoekstra H, and Guarino A (2017). Probiotics for gastrointestinal disorders: proposed recommendations for children of the Asia-Pacific region. World J Gastroenterol 23(45): 7952–7964. doi: 10.3748/wjg.v23.i45.7952
- Ghoshal UC, Gwee K-A, Holtmann G, Li Y, Park SJ, Simadibrata M, Sugano K, Wu K, Quigley EMM, and Cohen H (2018). The role of the microbiome and the use of probiotics in gastrointestinal disorders in adults in the Asia-Pacific region – background and recommendations of a regional consensus meeting. J Gastroenterol Hepatol 33(1): 57–69. doi: 10.1111/jgh.13840
- Salazar N, Arboleya S, Valdés L, Stanton C, Ross P, Ruiz L, Gueimonde M, Reyes-gavilán CGDL, Philip M, and Barnett G (2014). The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front Genet 5: 1–9. doi: 10.3389/fgene.2014.00406
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, and Versalovic J (2014). The placenta harbors a unique microbiome. Sci Transl Med 6(237): 1–11. doi: 10.1126/scitranslmed.3008599
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, and Rodríguez JM (2005). Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 51(4): 270–274. doi: 10.1007/s00284-005-0020-3
- DiGiulio DB (2012). Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med 17(1): 2–11. doi: 10.1016/j.siny.2011.10.001
- Wassenaar TM, and Panigrahi P (2014). Is a foetus developing in a sterile environment? Lett Appl Microbiol 59(6): 572–579. doi: 10.1111/lam.12334
- Moles L, Gómez M, Heilig H, Bustos G, Fuentes S, de Vos W, Fernández L, Rodríguez JM, and Jiménez E (2013). Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One 8(6): e66986. doi: 10.1371/journal.pone.0066986
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, and Rodríguez JM (2008). Is meconium from healthy newborns actually sterile? Res Microbiol 159(3): 187–193. doi: 10.1016/j.resmic.2007.12.007
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, Leite R, Elovitz MA, Parry S, and Bushman FD (2016). Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4(1):29. doi: 10.1186/s40168-016-0172-3
- Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, AND Walter J (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5(1):48. doi: 10.1186/s40168-017-0268-4
- Koenig JE, Spor A, Scalfone N, Fricker AD, Knight R, Angenent LT, Ley RE, Klaenhammer TR, Koenig JE, Spora A, Scalfone N, Fricker AD, Stombaughb J, Knightbc R, Angenentdf LT, and Ley RE (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108: 4578–4585. doi: 10.1073/pnas.1000081107
- Butel M (2014). Probiotics, gut microbiota and health. Med Mal Infect 44(1): 1–8. doi: 10.1016/j.medmal.2013.10.002
- Athalye-Jape G, Deshpande G, Rao S, and Patole S (2014). Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am J Clin Nutr 100(6): 1508–1519. doi: 10.3945/ajcn.114.092551
- Quin C, Estaki M, Vollman DM, Barnett JA, Gill SK, and Gibson DL (2018). Probiotic supplementation and associated infant gut microbiome and health: a cautionary retrospective clinical comparison. Sci Rep 8(1): 8283. doi: 10.1038/s41598-018-26423-3
- Cai J, Zhao C, Du Y, Zhang Y, Zhao M, and Zhao Q (2018). Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: systematic review with network meta-analysis. United Eur Gastroenterol J 6(2): 169–180. doi: 10.1177/2050640617736987
- Brown LM (2000). Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22(2): 283–97. doi: 10.1093/oxfordjournals.epirev.a018040
- Imase K, Tanaka A, Tokunaga K, Sugano H, Ishida H, and Takahashi S (2007). Lactobacillus reuteri tablets suppress Helicobacter pylori infection —A double-blind randomised placebo-controlled cross-over clinical study. J Japanese Assoc Infect Dis 81(4): 16–20. doi: 10.11150/kansenshogakuzasshi1970.81.387
- Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Bucci N, Canio A De, Indrio F, Cavallo L, Ierardi E, and Miniello VL (2008). Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy?: A pilot study. Helicobacter 13(2): 127–134. doi: 10.1111/j.1523-5378.2008.00593.x
- Onini FC, Rtolozzi FBA, Ducci FCAN, Elli MC, Ojetti V, Marota GCAM, Anti M, and Rini GGAS (2001). The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Alimenta 15(2): 163–169. doi: 10.1046/j.1365-2036.2001.00923.x
- Derwa Y, Gracie DJ, Hamlin PJ, and Ford AC (2017). Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 46(4): 389–400. doi: 10.1111/apt.14203
- Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, and Isolauri E (2001). Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357(9262): 1076–1079. doi: 10.1016/S0140-6736(00)04259-8
- Bieber T (2010). Atopic dermatitis. Ann Dermatol 22(2): 125–137. doi: 10.5021/ad.2010.22.2.125
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, and Williams H (2006). Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368(9537): 733–743. doi: 10.1016/S0140-6736(06)69283-0
- Nutten S (2015). Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66(1): 8–16. doi: 10.1159/000370220
- Fiset P-O, Leung DYM, and Hamid Q (2006). Immunopathology of atopic dermatitis. J Allergy Clin Immunol 118(1): 287–290. doi: 10.1016/j.jaci.2006.03.046
- McGirt LY, and Beck LA (2006). Innate immune defects in atopic dermatitis. J Allergy Clin Immunol 118(1): 202–208. doi: 10.1016/j.jaci.2006.04.033
- van der Aa LB, Heymans HSA, van Aalderen WMC, and Sprikkelman AB (2010). Probiotics and prebiotics in atopic dermatitis: review of the theoretical background and clinical evidence. Pediatr Allergy Immunol 21(2p2): e355–e367. doi: 10.1111/j.1399-3038.2009.00915.x
- Majamaa H, and Isolauri E (1996). Evaluation of the gut mucosal barrier: evidence for increased antigen transfer in children with atopic eczema. J Allergy Clin Immunol 97(4): 985–990. doi: 10.1016/s0091-6749(96)80074-1
- Majamaa H, and Isolauri E (1997). Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 99(2): 179–185. doi: 10.1016/s0091-6749(97)70093-9
- Laitinen K, Kalliomäki M, Poussa T, Lagström H, and Isolauri E (2005). Evaluation of diet and growth in children with and without atopic eczema: follow-up study from birth to 4 years. Br J Nutr 94(04): 565. doi: 10.1079/BJN20051503
- Simpson MR, Dotterud CK, Storrø O, Johnsen R, and Øien T (2015). Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol 15(1): 13. doi: 10.1186/s12895-015-0030-1
- Kopp M V., Hennemuth I, Heinzmann A, and Urbanek R (2008). Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 121(4): e850–e856. doi: 10.1542/peds.2007-1492
- Rather IA, Bajpai VK, Kumar S, Lim J, Paek WK, and Park Y-H (2016). Probiotics and atopic dermatitis: an overview. Front Microbiol 7: 507. doi: 10.3389/fmicb.2016.00507
- Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, and La Vecchia C (2012). Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23(3):402-14. doi: 10.1097/EDE.0b013e31824d5da2
- Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, Clerici M, Ricci E, Fusi A, De Vecchi E, Piconi S, and Drago L (2012). Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol 46: S33–S40. doi: 10.1097/MCG.0b013e31826a8468
- Huang R, Ning H, Shen M, Li J, Zhang J, and Chen X (2017). Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol 7:392. doi: 10.3389/fcimb.2017.00392
- Bilardi JE, Walker S, Temple-Smith M, McNair R, Mooney-Somers J, Bellhouse C, Fairley CK, Chen MY, and Bradshaw C (2013). The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One 8(9): e74378. doi: 10.1371/journal.pone.0074378
- Tandogdu Z, and Wagenlehner FME (2016). Global epidemiology of urinary tract infections. Curr Opin Infect Dis 29(1): 73–79. doi: 10.1097/QCO.0000000000000228
- Reid G (2017). The development of probiotics for women’s health. Can J Microbiol 63(4): 269–277. doi: 10.1139/cjm-2016-0733
- Petricevic L, Unger FM, Viernstein H, and Kiss H (2008). Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur J Obstet Gynecol Reprod Biol 141(1): 54–57. doi: 10.1016/j.ejogrb.2008.06.003
- Vujic G, Jajac Knez A, Despot Stefanovic V, and Kuzmic Vrbanovic V (2013). Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol 168(1): 75–79. doi: 10.1016/j.ejogrb.2012.12.031
- Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, and Geerlings SE (2012). Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double blind, noninferiority trial in postmenopausal women. Arch Intern Med 172(9): 704. doi: 10.1001/archinternmed.2012.777
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, and Busscher HJ (2011). Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9(1): 27–38. doi: 10.1038/nrmicro2473
- Karlsson M, Scherbak N, Khalaf H, Olsson P-E, and Jass J (2012). Substances released from probiotic Lactobacillus rhamnosus GR-1 potentiate NF-κB activity in Escherichia coli -stimulated urinary bladder cells. FEMS Immunol Med Microbiol 66(2): 147–156. doi: 10.1111/j.1574-695X.2012.00994.x
- Köhler GA, Assefa S, and Reid G (2012). Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol 2012: 1–14. doi: 10.1155/2012/636474
- Martinez RCR, Seney SL, Summers KL, Nomizo A, De Martinis ECP, and Reid G (2009). Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol Immunol 53(9): 487–495. doi: 10.1111/j.1348-0421.2009.00154.x
- Martinez RCR, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, De Martinis ECP, and Reid G (2009). Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol 48(3): 269–274. doi: 10.1111/j.1472-765X.2008.02477.x
- Verdenelli MC, Cecchini C, Coman MM, Silvi S, Orpianesi C, Coata G, Cresci A, and Di Renzo GC (2016). Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr Microbiol 73(4): 483–490. doi: 10.1007/s00284-016-1085-x
- Rossi A, Rossi T, Bertini M, and Caccia G (2010). The use of Lactobacillus rhamnosus in the therapy of bacterial vaginosis. Evaluation of clinical efficacy in a population of 40 women treated for 24 months. Arch Gynecol Obstet 281(6): 1065–1069. doi: 10.1007/s00404-009-1287-6
- Petricevic L, and Witt A (2008). The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. Gen Gynaecol 1369–1374. doi: 10.1111/j.1471-0528.2008.01882.x
- Larsson P-G, Brandsborg E, Forsum U, Pendharkar S, Andersen KK, Nasic S, Hammarström L, and Marcotte H (2011). Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect Dis 11(1): 223. doi: 10.1186/1471-2334-11-223
- Petrova MI, Macklaim JM, Wuyts S, Verhoeven T, Vanderleyden J, Gloor GB, Lebeer S, and Reid G (2018). Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front Microbiol 9: 1278. doi: 10.3389/fmicb.2018.01278
- Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR, Chaudhry R, Chen HH, Johnson JA, Morris JG, Paneth N, and Gewolb IH (2017). A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548(7668): 407–412. doi: 10.1038/nature23480
- Athalye-Jape G, Rao S, and Patole S (2018). Effects of probiotics on experimental necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Res 83(1–1): 16–22. doi: 10.1038/pr.2017.218
- Sawh SC, Deshpande S, Jansen S, Reynaert CJ, and Jones PM (2016). Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 4: e2429. doi: 10.7717/peerj.2429
- Kochan P, Chmielarczyk A, Szymaniak L, Brykczynski M, Galant K, Zych A, Pakosz K, Giedrys-Kalemba S, Lenouvel E, and Heczko PB (2011). Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—is the time right to revise probiotic safety guidelines? Clin Microbiol Infect 17(10):1589-92. doi: 10.1111/j.1469-0691.2011.03614.x
- Billimoria ZC, Pandya S, Bhatt P, and Pandya B (2016). Probiotics—to use, or not to use? An updated meta-analysis. Clin Pediatr 55(13): 1242–1244. doi: 10.1177/0009922816664067
- McFarland L V., Evans CT, and Goldstein EJC (2018). Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med 5: 124. doi: 10.3389/fmed.2018.00124
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB-Z, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, and Elinav E (2018). Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174(6): 1388–1405.e21. doi: 10.1016/j.cell.2018.08.041
- Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, and Elinav E (2018). Post-Antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174(6): 1406–1423.e16. doi: 10.1016/j.cell.2018.08.047
- Gallagher J (2018). Probiotics labelled “quite useless.” BBC. Available at; https://www.bbc.com/news/health-45434753 [Accessed 01.10.2018].
- Daniells S (2018). No BBC, probiotics are not “quite useless.” Nutraingredients-USA. Available at: https://www.nutraingredients-usa.com/Article/2018/09/07/No-BBC-probiotics-are-not-quite-useless [Accessed 30.10.2018].
- Reid G (2018). Trying to close the stable door after the horse has bolted. Microbiome Times. Available at: http://www.microbiometimes.com/trying-to-close-the-stable-door-after-the-horse-has-bolted/ [Accessed 01.10.2018].
- ISAPP Board of Directors, Guarner F, and Pot B (2018). Clinical evidence and not microbiota outcomes drive value of probiotics. Available at: https://isappscience.org/clinical-evidence-not-microbiota-outcomes-drive-value-probiotics/ [Accessed 20.10.2018].
- Park C, Brietzke E, Rosenblat JD, Musial N, Zuckerman H, Ragguett R-M, Pan Z, Rong C, Fus D, and McIntyre RS (2018). Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism? Brain Behav Immun 73: 115–124. doi: 10.1016/j.bbi.2018.07.006
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, Stanley T, Abels P, Purdie G, Maude R, and Mitchell EA (2017). Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine.24: 159–165. doi: 10.1016/j.ebiom.2017.09.013
- Wang L, Guo M-J, Gao Q, Yang J-F, Yang L, Pang X-L, and Jiang X-J (2018). The effects of probiotics on total cholesterol. Medicine 97(5): e9679. doi: 10.1097/MD.0000000000009679
- Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, and Stanton C (2017). Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun 59: 21–37. doi: 10.1016/j.bbi.2016.07.145
- Ogawa J, Kishino S, Ando A, Sugimoto S, Mihara K, and Shimizu S (2005). Production of conjugated fatty acids by lactic acid bacteria. J Biosci Bioeng 100(4): 355–364. doi: 10.1263/jbb.100.355
- Lyte M, and Brown DR (2018). Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of Lactobacillus: Implications for interkingdom communication within the microbiota-gut-brain axis. PLoS One 13(1): e0191037. doi: 10.1371/journal.pone.0191037
- Patel A, Shah N, and Prajapati JB (2013). Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera – a promising approach. Croat J Food Sci Technol 5(2): 85–91.
- Ceccarelli G, Fratino M, Selvaggi C, Giustini N, Serafino S, Schietroma I, Corano Scheri G, Pavone P, Passavanti G, Alunni Fegatelli D, Mezzaroma I, Antonelli G, Vullo V, Scagnolari C, and D’Ettorre G (2017). A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav 7(8): e00756. doi: 10.1002/brb3.756
- Phelps DL, Balog J, Gildea LF, Bodai Z, Savage A, El-Bahrawy MA, Speller AV, Rosini F, Kudo H, McKenzie JS, Brown R, Takáts Z, and Ghaem-Maghami S (2018). The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Br J Cancer 118(10): 1349–1358. doi: 10.1038/s41416-018-0048-3
- Ji X, Wang H, Song B, Chu B, and He Y (2018). Silicon nanomaterials for biosensing and bioimaging analysis. Front Chem 6: 38. doi: 10.3389/fchem.2018.00038
- He J, Zhang F, and Han Y (2017). Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes. Medicine 96(51): e9166. doi: 10.1097/MD.0000000000009166
- Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, Brigidi P, and Gibson GR (2017). An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One 12(12): e0187964. doi: 10.1371/journal.pone.0187964
- Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, and Baker JE (2012). Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26(4): 1727–1735. doi: 10.1096/fj.11-197921
- Gan XT, Ettinger G, Huang CX, Burton JP, Haist J V., Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, and Karmazyn M (2014). Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Hear Fail 7(3): 491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978
- Miyazaki K, Masuoka N, Kano M, and Iizuka R (2014). Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef Microbes 5(2): 121–128. doi: 10.3920/BM2012.0066
- Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Wong RG, Urquhart BL, Dinculescu V, Ruetz KN, Velenosi TJ, Pignanelli M, and Spence JD (2018). Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 273: 91–97. doi: 10.1016/j.atherosclerosis.2018.04.015
- United Nations Environment. Available at: https://www.unenvironment.org/explore-topics/water/what-we-do/tackling-global-water-pollution. [Accessed 22.07.2018].
- Spanogiannopoulos P, Bess EN, Carmody RN, and Turnbaugh PJ (2016). The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14(5): 273–287. doi: 10.1038/nrmicro.2016.17
- Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, and Reid G (2014). Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. MBio 5(5): e01580-14. doi: 10.1128/mBio.01580-14
- Nowak A, Kuberski S, and Libudzisz Z (2014). Probiotic lactic acid bacteria detoxify N-nitrosodimethylamine. Food Addit Contam Part A 31(10): 1678–1687. doi: 10.1080/19440049.2014.943304
- Nduti N, McMillan A, Seney S, Sumarah M, Njeru P, Mwaniki M, and Reid G (2016). Investigating probiotic yoghurt to reduce an aflatoxin B1 biomarker among school children in eastern Kenya: preliminary study. Int Dairy J 63: 124–129. doi: 10.1016/j.idairyj.2016.07.014
- aus der Beek T, Weber F-A, Bergmann A, Hickmann S, Ebert I, Hein A, and Küster A (2016). Pharmaceuticals in the environment-global occurrences and perspectives. Environ Toxicol Chem 35(4): 823–835. doi: 10.1002/etc.3339
- Global Market Insights Inc (2017). Probiotics Market Report, 2024. Delaware. Available at: https://www.gminsights.com/industry-analysis/probiotics-market [Accessed: 13.11.2018]
- Whorwell PJ, Altringer L, Morel J, Ph D, Bond Y, Charbonneau D, Ph D, Mahony LO, Ph D, Kiely B, Ph D, Shanahan F, and Quigley EMM (2006). Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101: 1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x
- Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier C-H, and Matuchansky C (2007). Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther 26(3): 475–486. doi: 10.1111/j.1365-2036.2007.03362.x
- Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Feuillerat NG, Schlumberger A, Jakob S, and Whorwell PJ (2008). Clinical trial?: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther 29: 104–114. doi: 10.1111/j.1365-2036.2008.03853.x
- Yang Y, He M, Hu G, Wei J, Pages P, Yang X, and Bourdu-Naturel S (2008). Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol 14(40): 6237–6243. doi: 10.3748/wjg.14.6237
- Trial P, Ojetti V, Ianiro G, Tortora A, Angelo GD, Antonella T, Rienzo D, Bibbò S, Migneco A, and Gasbarrini A (2014). The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation?: a randomized, double-blind, placebo-controlled trial. J Gastrointest Liver Dis 23(4): 387–391. doi: 10.15403/jgld.2014.1121.234.elr
- Sampalis J, Psaradellis E, and Rampakakis E (2010). Efficacy of BIO K + CL1285 ® in the reduction of antibiotic-associated diarrhea – a placebo controlled double-blind randomized, multi-center study. Arch Med Sci 6(1): 56–64. doi: 10.5114/aoms.2010.13508
- Beausoleil M, Fortier N, Guenette S, L’Ecuyer A, Savoie M, Franco M, Lachaine J, and Weis K (2007). Effect of a fermented milk combining Lactobacillus acidophilus CL1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol 21(11): 732–736. doi: 10.1155/2007/720205
- Cimperman L, Bayless G, Best K, Diligente A, Mordarski B, Oster M, Smith M, Vatakis F, Wiese D, Steiber A, and Katz J (2011). Study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol 45(9): 785–789.
- Kale-pradhan PB, Pharm D, Jassal HK, Wilhelm SM, and Pharm D (2010). Role of Lactobacillus in the prevention of antibiotic-associated diarrhea?: a meta-analysis. Pharmacotherapy 30(2): 119–126. doi: 10.1592/phco.30.2.119
- Szajewska H, and Kołodziej M (2015). Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther 42(7): 793–801. doi: 10.1111/apt.13344
- Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, and Bruce AW. (2003). Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol 35: 131-134. doi: 10.1016/s0928-8244(02)00465-0
- Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, and Reid G (2006). Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect 8(12–13): 2772–2776. doi: 10.1016/j.micinf.2006.08.008
- Marcone V, Rocca G, Lichtner M, and Calzolari E (2010). Long-term vaginal administration of Lactobacillus rhamnosus as a complementary approach to management of bacterial vaginosis. Int J Gynecol Obstet 110(3): 223–226. doi: 10.1016/j.ijgo.2010.04.025
- Probiotic guides and level of evidence. (2018). Available at: www.usprobioticguide.com; www.probioticchart.ca [Accessed: 19.11.2018]
ACKNOWLEDGMENTS
The support of NSERC and the Mexi-can National Council for Science and Technology (CONACYT) for SPB is appreciated.
COPYRIGHT
© 2019

Forty-five-year evolution of probiotic therapy by Puebla-Barragan and Reid is licensed under a Creative Commons Attribution 4.0 International License.