Back to article: Microfluidic techniques for separation of bacterial cells via taxis
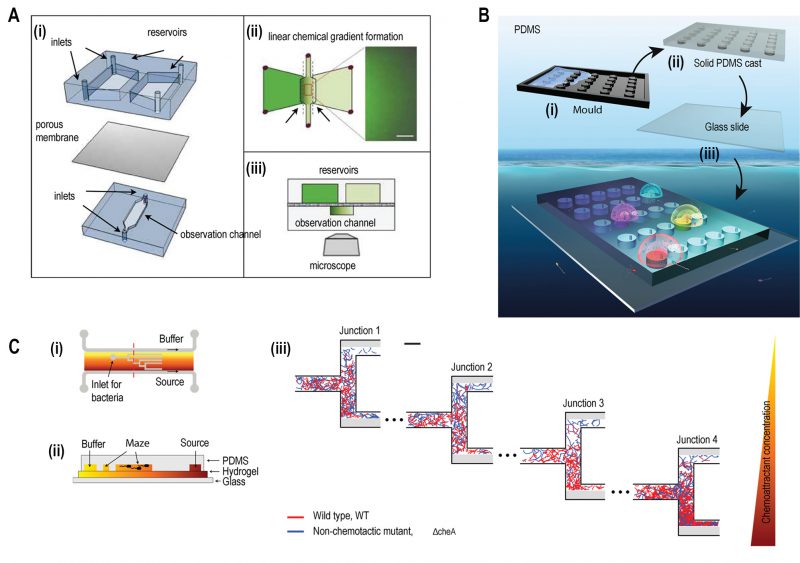
FIGURE 2: Microfluidic devices for bacterial chemotaxis (static conditions). (A) (i) A two-layer PDMS cast was separated by aluminium oxide porous membrane. The top layer contains two reservoirs; one with chemo effector and other with standard buffer whereas the bottom layer contains the observation channel loaded with bacterial culture. (ii) A linear chemical gradient was generated by diffusion across the porous membrane into the observation channel. (iii) An inverted microscope objective lens was mounted below the observation channel for visualising bacteria to monitor motility and chemotactic response. Reproduced from [27]. (B) In Situ Chemotaxis Assay (ISCA): (i) Mould was fabricated by 3D printing. (ii) PDMS was cast over the mould. (iii) PDMS was peeled off and bonded to a glass slide to create cylindrical chambers. (iv) Drops of bacterial solutions from ocean samples were placed over PDMS chambers which were interconnected by small pores. Reproduced from < [29]. (C) T-maze for sorting chemotactic motile bacteria. (i) Top view of the microfluidic chip, consisting of hydrogel-filled T-shaped microchannels with end for bacterial inlet and flanked by channels for a source containing chemo effectors at one side and buffer at the other. (ii) A cross-sectional view of the chip fabricated by hydrogel-PDMS hybrid with increasing concentration of chemo effectors across the maze represented by increasing from yellow to orange colour. (iii) Wild-type chemotactic motile bacteria (red) get collected in the lower end of Junction 4 (region of high chemoattractant concentration) whereas non-chemotactic mutant (blue) gets collected in the upper-end Junction 1 (region of low chemoattractant concentration). Reproduced from [31].
27. Nagy K, Sipos O, Valkai S, Gombai É, Hodula O, Kerényi Á, Ormos P, and Galajda P (2015). Microfluidic study of the chemotactic response of Escherichia coli to amino acids, signaling molecules and secondary metabolites. Biomicrofluidics 9(4): 044105. doi: 10.1063/1.4926981
29. Lambert BS, Raina J-B, Fernandez VI, Rinke C, Siboni N, Rubino F, Hugenholtz P, Tyson GW, Seymour JR, and Stocker R (2017). A microfluidics-based in situ chemotaxis assay to study the behaviour of aquatic microbial communities. Nat Microbiol 2(10): 1344–1349. doi: 10.1038/s41564-017-0010-9
31. Salek MM, Carrara F, Fernandez V, Guasto JS, and Stocker R (2019). Bacterial chemotaxis in a microfluidic T-maze reveals strong phenotypic heterogeneity in chemotactic sensitivity. Nat Commun 10(1): 1877. doi: 10.1038/s41467-019-09521-2