Back to article: Milestones in Bacillus subtilis sporulation research
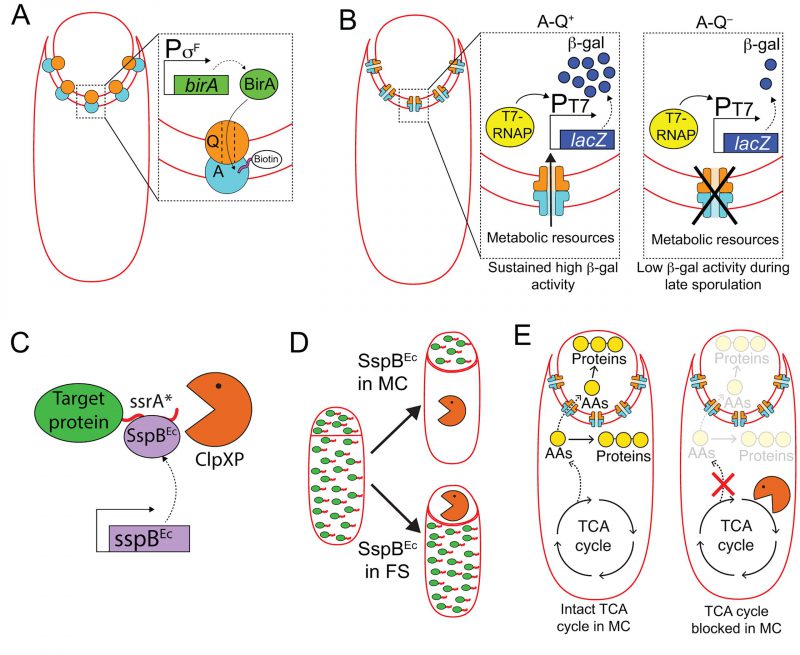
FIGURE 6: Harnessing cell-specific gene expression to study the function of A-Q complexes. (A) The forespore protein SpoIIQ (Q, orange circle) and the mother-cell proteins encoded in the spoIIIA operon (A, light blue circle), form trans-envelope complexes that bridge the forespore and mother-cell membranes during engulfment. Zoomed in panel: Biotin ligase (BirA, green) produced in the forespore is able to biotinylate a biotin acceptor peptide (pink) fused to the extracellular domain of the A protein, SpoIIIAH [111], indicating that A-Q complexes are channels (see panel B), and that the channel pore is open on the forespore side and large enough for BirA to reach the extracellular domain of SpoIIIAH. (B) The activity of a heterologous RNA polymerase (T7 RNAP, yellow) produced in the forespore under σF control is monitored by the accumulation of β-galactosidase (β-gal, dark blue circles) produced through the expression of a lacZ gene under the control of a T7 RNAP-dependent promoter. In the presence of A-Q channels (A-Q+, left zoomed in panel), sustained β-galactosidase activity is detected throughout sporulation. In the absence of A-Q channels (A-Q−, right zoomed in panel), β-galactosidase activity drops at later sporulation stages. Camp and Losick [112] proposed that A-Q channels constitute feeding tubes through which the mother cell transfers metabolic resources to the forespore to maintain biosynthetic activities at late sporulation stages. (C) The ssrA*/SspBEc inducible protein degradation system [114]. SspBEc (purple) binds to ssrA* (red) fused to the C-terminus of target proteins (green), and delivers the target proteins to the endogenous B. subtilis protease ClpXP (orange pacman) for degradation. (D) Cell-specific degradation of target proteins during sporulation is achieved by expressing sspBEc from mother cell- or forespore-specific promoters [113]. Target proteins represented by green ovals tagged with ssrA (red line); degradation represented by orange pacman. (E) Left cell: Mother-cell TCA cycle provides metabolic precursors, such as amino acids (AAs, yellow circles), to support protein synthesis in both the mother cell and the forespore [115]. Mother-cell metabolic precursors could be transported to the forespore via A-Q channels, in keeping with the feeding tube model. Right cell: Degradation of TCA cycle enzymes in the mother cell blocks protein synthesis in both the mother cell and the forespore [115].
111. Meisner J, Wang X, Serrano M, Henriques AO, and Moran CP (2008). A channel connecting the mother cell and forespore during bacterial endospore formation. Proc Natl Acad Sci U S A 105(39): 15100–5. 10.1073/pnas.0806301105
112. Camp AH, and Losick R (2009). A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev 23(8): 1014–24. 10.1101/gad.1781709
113. Riley EP, Trinquier A, Reilly ML, Durchon M, Perera VR, Pogliano K, and Lopez-Garrido J (2018). Spatiotemporally regulated proteolysis to dissect the role of vegetative proteins during Bacillus subtilis sporulation: cell-specific requirement of σH and σA. Mol Microbiol 108(1): 45–62. 10.1111/mmi.13916
114. Griffith KL, and Grossman AD (2008). Inducible protein degradation in Bacillus subtilis using heterologous peptide tags and adaptor proteins to target substrates to the protease ClpXP. Mol Microbiol 70(4): 1012–25. 10.1111/j.1365-2958.2008.06467.x
115. Riley EP, Lopez-Garrido J, Sugie J, Liu RB and Pogliano K (2020). Metabolic differentiation and intercellular nurturing underpin bacterial endospore formation. Science Advances. In press.