Back to article: Urm1, not quite a ubiquitin-like modifier?
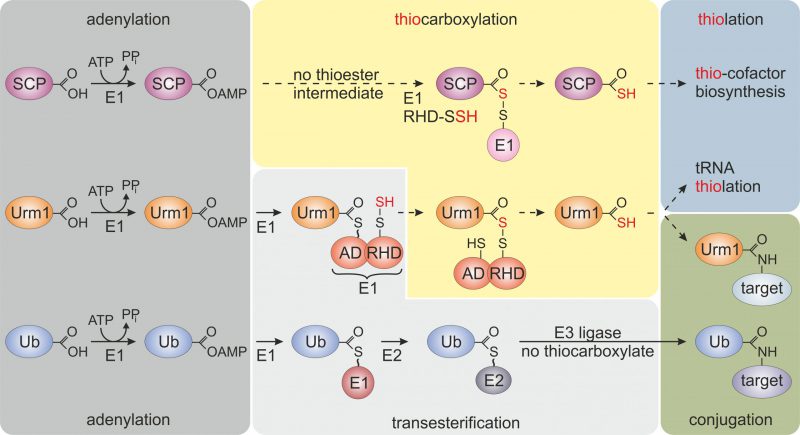
FIGURE 1: Selected members of the ubiquitin-fold protein family. Prokaryotic sulfur carrier proteins (SCP) as well as eukaryotic Urm1 and ubiquitin (Ub) all require activating adenylation by E1-type enzymes. For the Ub pathway, E1 activation results in an E1~Ub thioester that is passed onto E2/E3 enzymes via transesterifications and eventually conjugated to lysine residues in target proteins. By contrast, E1-type adenylation of SCP does not follow a thioester intermediate; rather a dedicated desulfurase and rhodanese domain (RHD) protein (i.e., IscS, not shown) engages in sulfur transfer, persulfidation (-SSH) and eventually, thiocarboxylation (SCP-COSH) for use of SCP as a sulfur donor in thiolation reactions including thio-cofactor synthesis [4][21]. As for Urm1, following adenylation a thioester is formed to the adenylation domain (AD) of Uba4, the E1-type enzyme for Urm1, and passed over to a persulfide (-SSH) on the RHD of the same enzyme. Persulfidation of the latter requires sulfur mobilization from cysteine by desulfurase Nfs1 (not shown) and direct S-transfer to the RHD in Uba4 or indirectly via sulfotransferase Tum1 (not shown) [11][14][17][18]. Next, from the formed acyl-disulfide, reductive cleavage (not shown) releases the Urm1 thiocarboxylate (Urm1-COSH) for S-transfer in downstream tRNA thiolation reactions. Urm1-COSH also operates in urmylation, a non-canonical, lysine-directed protein conjugation thought to be similar to ubiquitylation [17][18][19][21].
4. Kessler D (2006). Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol Rev 30(6): 825–840. 10.1111/j.1574-6976.2006.00036.x
11. Leidel S, Pedrioli PGA, Bucher T, Brost R, Costanzo M, Schmidt A, Aebersold R, Boone C, Hofmann K, and Peter M (2009). Ubiquitin-related modifier Urm1 functions as a sulphur-carrier in thiolation of eukaryotic tRNA. Nature 458(7235): 228–232. 10.1038/nature07643
14. Jüdes A, Bruch A, Klassen R, Helm, M and Schaffrath R (2016). Sulfur transfer and activation by ubiquitin-like modifier system Uba4•Urm1 link protein urmylation and tRNA thiolation in yeast. Microb Cell 3(11): 554–564. 10.15698/mic2016.11.539
17. Termathe M, and Leidel SA (2018). The Uba4 domain interplay is mediated via a thioester that is critical for tRNA thiolation through Urm1 thiocarboxylation. Nucleic Acids Res 46(10): 5171–5181. 10.1093/nar/gky312
18. Pabis M; Termathe M, Ravichandran KE, Kienast SD, Krutyhołowa R, Sokołowski M, Jankowska U, Grudnik P, Leidel SA, and Glatt S (2020). Molecular basis for the bifunctional Uba4-Urm1 sulfur-relay system in tRNA thiolation and ubiquitin-like conjugation. EMBO J 39(19): e105087. 10.15252/embj.2020105087
19. Van der Veen AG, Schorpp K, Schlieker C, Buti L, Damon JR, Spooner E, Ploegh H, and Jentsch S (2011). Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc Natl Acad Sci USA 108(5): 1763–1770. 10.1073/pnas.1014402108
21. Termathe M, and Leidel SA (2021). Urm1: A Non-Canonical UBL. Biomolecules 11(2):139. 10.3390/biom11020139