Back to article: Pirates of the haemoglobin
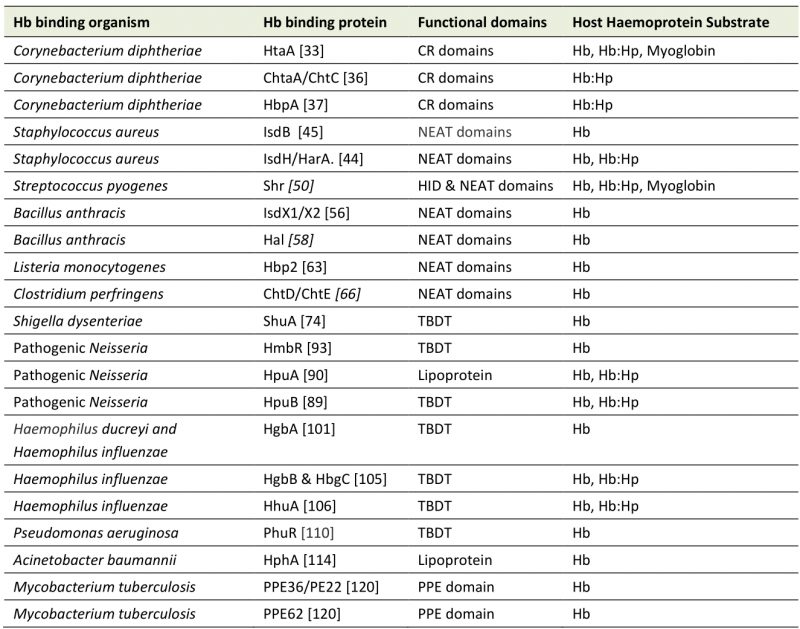
TABLE 2. Haemoglobin binding proteins in human pathogens. Human pathogens that use Hb as an iron source usually bind Hb through specific surface exposed membrane proteins, extracellular lipoproteins or secreted haemophores. Pathogens can have more than one Hb binding system and these proteins can have single or multiple substrates.
33. Allen CE, and Schmitt MP (2009). HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtherial. J Bacteriol 191(8): 2638–2648. 10.1128/JB.01784-08
36. Allen CE, and Schmitt MP (2015). Utilization of host iron sources by Corynebacterium diphtheriae: Multiple hemoglobin-binding proteins are essential for the use of iron from the hemoglobin-haptoglobin complex. J Bacteriol 197(3): 553–562. 10.1128/JB.02413-14
37. Lyman LR, Peng ED, and Schmitt MP (2018). Corynebacterium diphtheriae Iron-Regulated Surface Protein HbpA Is Involved in the Utilization of the Hemoglobin-Haptoglobin Complex as an Iron Source. J Bacteriol 200(7): e00676-17. 10.1128/JB.00676-17
44. Pilpa RM, Robson SA, Villareal VA, Wong ML, Phillips M, and Clubb RT (2009). Functionally distinct NEAT (NEAr Transporter) domains within the staphylococcus aureus IsdH/HarA protein extract heme from methemoglobin. J Biol Chem 284(2): 1166–1176. 10.1074/jbc.M806007200
45. Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, and Lei B (2008). Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem 283(26): 18450–18460. 10.1074/jbc.M801466200
50. Bates CS, Montañez GE, Woods CR, Vincent RM, and Eichenbaum Z (2003). Identification and Characterization of a Streptococcus pyogenes Operon Involved in Binding of Hemoproteins and Acquisition of Iron. Infect Immun 71(3): 1042–1055. 10.1128/IAI.71.3.1042-1055.2003
56. Tarlovsky Y, Fabian M, Solomaha E, Honsa E, Olson JS, and Maresso AW (2010). A Bacillus anthracis S-Layer Homology Protein That Binds Heme and Mediates Heme Delivery to IsdC. J Bacteriol 192(13): 3503–3511. 10.1128/JB.00054-10
58. Balderas MA, Nobles CL, Honsa ES, Alicki ER, and Maresso AW (2012). Hal is a Bacillus anthracis heme acquisition protein. J Bacteriol 194(20): 5513–5521. 10.1128/JB.00685-12
63. Malmirchegini GR, Sjodt M, Shnitkind S, Sawaya MR, Rosinski J, Newton SM, Klebba PE, and Clubb RT (2014). Novel mechanism of hemin capture by Hbp2, the hemoglobin-binding hemophore from Listeria monocytogenes. J Biol Chem 289(50): 34886–34899. 10.1074/jbc.M114.583013
66. Choo JM, Cheung JK, Wisniewski JA, Steer DL, Bulach DM, Hiscox TJ, Chakravorty A, Smith AI, Gell DA, Rood JI, and Awad MM (2016). The NEAT Domain-Containing Proteins of Clostridium perfringens Bind Heme. PLOS ONE 11(9): e0162981. 10.1371/journal.pone.0162981
74. Burkhard KA, and Wilks A (2007). Characterization of the outer membrane receptor ShuA from the heme uptake system of Shigella dysenteriae: Substrate specificity and identification of the heme protein ligands. J Biol Chem 282(20): 15126–15136. 10.1074/jbc.M611121200
89. Rohde KH, and Dyer DW (2004). Analysis of Haptoglobin and Hemoglobin-Haptoglobin Interactions with the Neisseria meningitidis TonB-Dependent Receptor HpuAB by Flow Cytometry. Infect Immun 72(5): 2494–2506. 10.1128/IAI.72.5.2494-2506.2004
90. Wong CT, Xu Y, Gupta A, Garnett JA, Matthews SJ, and Hare SA (2015). Structural analysis of haemoglobin binding by HpuA from the Neisseriaceae family. Nat Commun 6: 1–11. 10.1038/ncomms10172
93. Perkins-Balding D, Baer MT, and Stojiljkovic I (2003). Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology 149(12): 3423–3435. 10.1099/mic.0.26448-0
101. Al-Tawfiq JA, Fortney KR, Katz BP, Hood AF, Elkins C, and Spinola SM (2000). An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J Infect Dis.181(3): 1049–1054. 10.1086/315309
105. Maciver I, Latimer JL, Liem HH, Muller-Eberhard U, Hrkal Z, and Hansen EJ (1996). Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect Immun 64(9): 3703–3712. 10.1128/IAI.64.9.3703-3712.1996
106. Cope LD, Hrkal Z, and Hansen EJ (2000). Detection of phase variation in expression of proteins involved in hemoglobin and hemoglobin-haptoglobin binding by nontypeable Haemophilus influenzae. Infect Immun 68(7): 4092–4101. 10.1128/iai.68.7.4092-4101.2000
110. Marvig RL, Damkiær S, Hossein Khademi SM, Markussen TM, Molin S, and Jelsbak L (2014). Within-host evolution of pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. mBio 5(3): e00966-14. 10.1128/mBio.00966-14
114. Bateman TJ, Shah M, Ho TP, Shin HE, Pan C, Harris G, Fegan JE, Islam EA, Ahn SK, Hooda Y, Gray-Owen SD, Chen W, and Moraes TF (2021). A Slam-dependent hemophore contributes to heme acquisition in the bacterial pathogen Acinetobacter baumannii. Nat Commun 12: 6270. 10.1038/s41467-021-26545-9
120. Mitra A, Speer A, Lin K, Ehrt S, and Niederweis M (2017). PPE surface proteins are required for heme utilization by Mycobacterium tuberculosis. mBio 8(1): e01720-16. 10.1128/mBio.01720-16