Research Articles:
Microbial Cell, Vol. 9, No. 5, pp. 103 - 122; doi: 10.15698/mic2022.05.776
Air- and dustborne fungi in repositories of the National Archive of the Republic of Cuba
1 Conservation Preventive Laboratory, National Archive of the Republic of Cuba, Havana, Cuba.
Keywords: airborne fungi, dustborne fungi, indoor environment, environmental quality, repositories, archive, biodeterioration.
Abbreviations:
AEI – amylolytic EI,
CEI – cellulolytic EI,
CFU – colony forming unit,
EI – enzymatic index,
I/O – indoor/outdoor,
NARC – National Archive of the Republic Cuba,
PEI – proteolytic EI,
PNSM – pigmented non-sporulating mycelia,
QS – Sørensen's coefficient of similarity,
RD – relative density,
RF – relative frequency,
WNSM – white non-sporulating mycelia.
Received originally: 11/09/2021 Received in revised form: 25/02/2022
Accepted: 07/04/2022
Published: 01/04/2022
Correspondence:
Sofia Borrego, Conservation Preventive Laboratory, National Archive of the Republic of Cuba, Compostela No. 906 esq a San Isidro, Habana Vieja, PO Box: 10100, Havana, Cuba; Phone: +53 7862 9436; Fax: +53 7866 8080; ORCID: 0000-0001-8739-2577; sofy.borrego@gmail.com
Conflict of interest statement: Authors declare that they have no conflict of interest.
Please cite this article as: Sofia Borrego, Isbel Vivar and Alian Molina (2022). Air- and dustborne fungi in repositories of the National Archive of the Republic of Cuba. Microbial Cell 9(5): 103-122. doi: 10.15698/mic2022.05.776
Abstract
This study has as objectives to determine the concentration and diversity of the air- and dustborne mycobiota in seven National Archive of the Republic of Cuba repositories, and to assess the potential risk of biodeterioration that isolated taxa may have. In the indoor and outdoor environmental microbiological samplings a SAS biocollector was used and the indoor/outdoor (I/O) ratio was determined for each repository. The settled dust was collected during six months. Sørensen’s coefficient of similarity (QS) was calculated to compare the isolated taxa among the three studied niches (indoor air, dust, outdoor air). The biodegradation potential of the isolated taxa was determined by semi-quantitative tests. The concentrations in the air of repositories with natural cross-ventilation ranged from 225.2-750.3 CFU m-3, while in the Map library with air-conditioning the concentration was significantly lower. The I/O ratios ranged from 0.1-1.7 revealing different environmental qualities. The maximum settled dust load was 22.8 mg/m2/day with a top fungal concentration of 6000 CFU g-1. 14 and eleven genera were detected in the air and dust respectively with predominance of the genera Aspergillus, Cladosporium and Penicillium. A QS of 0.8 was obtained between the indoor and the outdoor environments with eleven taxa similar evidencing the incidence of outdoors on the indoor mycobiota. The isolated taxa showed several biodeteriogenic attributes highlighting twelve and 14 taxa from indoor air and dust respectively with positive results for the five tests performed. This demonstrates the potential risk that fungal environmental represent for the preserved documentary heritage.
INTRODUCTION
In outdoor and indoor environments there is a large number of biotic and abiotic particles of different origin, shape and size suspended in the air, constituting the atmospheric aerosol. They can be classified in different ways considering the origin (biological, organic, inorganic), the location (marine, continental, rural, industrial, urban) and the effect they may have on the surfaces where they settled (chemical, toxic, pathogenic, degrading). Aerosols of biological origin or bioaerosols are particles of microscopic size suspended in the air that can affect human beings causing allergy, toxicity or infection. They can be constituted by protozoa, pollens, microalgae, propagules of multicellular fungi (spores, hyphae, fragments of these and other structures), yeasts (unicellular fungi), bacteria, viruses, toxins and in general any fragment of microorganisms with diverse aerodynamic diameters that comprise dimensions ranging from the order of the nanometers to about 1 mm [1][2].
–
Among the components of the atmospheric aerosol, there is also dust. Hence microorganisms can be found freely in the airborne state or associated with dust particles that can settle on the surface of materials [3]. On indoor environments, the air and dust may represent two different niches [4].
–
Dust is considered an important transport medium for the spread of microorganisms such as bacteria, fungi and spores, as well as minerals and fine particles [5]. This dust can deposit on documents, books, artworks and on many other objects; it varies in quantity and quality depending on the situation of the building, the activities that take place inside, the season of the year, the type of ventilation/air conditioning and the state of conservation of the building [6][7]. The microbial concentration in the dust reflects the microbial accumulation of a longer period than can be obtained by making a punctual air sampling, which is why it is a matrix that serves as a reservoir of fungal contamination in indoor environments [4][8]. In this way, when performing the microbial evaluation of the accumulated dust, the behavior of the microbiota during a long period of exposure is being determined. Additionally, dust serves as a source of nutrients for some insects and microorganisms, the latter can also be transported by their particles to the interior of the facilities through ventilation/air-conditioning systems and by people [7][9]. In archives, libraries and museums, the dust sedimentation can create a microenvironment on the artworks surfaces and the documentary collections that prevents the normal flow of air over them, facilitating the absorption of moisture and favoring the growth and development of microorganisms, mainly of filamentous fungi [10][11]. Those can generate biofilms on the materials on which they are settled, representing a significant risk of microbial biodeterioration for important documentary collections and art pieces. In this way, the dust constitutes a reservoir of fungal propagules that resupply the air when there is activity in the premises and that can be inhaled [12]. Although fungal propagules are easily dispersed and transported through air, dust, insects, and people [13], fungal growth and colonization on the artwork surfaces and documents found within archives, libraries and museums can also be an important source of air pollution, either with their re-suspended propagules, volatile organic compounds or mycotoxins that are products of their metabolism [4][9][14][15]. For that reason, the assessment of airborne and dustborne fungi provides important information on the mycological quality of indoor environments. Hence, some authors have isolated microorganisms and particularly fungi, both from air and from dust settled in archives, libraries and museums [11][14][16][17][18].
–
On the other hand, it is known that paper and other documentary materials (microforms, films, audiovisuals, etc.) are an excellent substrate for various heterotrophic organisms, especially fungi, because these materials contain different organic substances such as cellulose or cellulose derivate compounds, glues, inks, pigments and fillers of animal origin [17][19][20]. Fungi can easily grow on these materials, biodegrading them [13][19][21]. In addition, environmental fungi can be a risk for human health since some species are also potentially pathogenic, causing allergy, infections and toxic effects [9][22][23]. The study of indoor microbial contamination in archives, libraries and museums is of great interest to conservators, restorers and researchers due to the impact represented by microorganisms and especially fungi in the deterioration of artifacts and in human health [14][16][19][20][24][25][26][27][28].
–
In the National Archive of the Republic of Cuba (NARC) studies have been carried out for years where the fungal concentrations in the air from different repositories have been evaluated [20][26][29][30][31][32][33] as well as the presence of fungi on different types of documents made of various materials [20][24][34][35]. However, studies on the fungal characterization of settled dust are very scarce [36][37]. Therefore, this study has as objectives to determine the concentration and diversity of the air- and dustborne mycobiota in seven NARC repositories, and to assess the potential risk of biodeterioration that isolated taxa may have.
RESULTS
Concentration and distribution of airborne fungi in the environment of the analyzed repositories
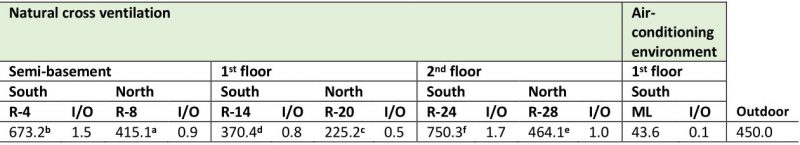
Fungal concentration obtained in the repositories with natural cross-ventilation ranged between 225.2 colony forming units (CFU) m−3 and 750.3 CFU m−3, while in the Map library, which has air conditioning, concentration was significantly lower (p ≤ 0.01), i.e., only 43.6 CFU m−3. On the other hand, the recorded concentrations in the repositories located on the south side of the building were significantly higher than those obtained in the repositories located on the north side (Table 1). When comparing the fungal concentrations obtained per floor, it was observed that the first floor showed the lowest values (R-14 = 370.4 CFU m−3 and R-20 = 225.2 CFU m−3) while in the second floor the values were highest (R-24 = 750.3 CFU m−3 and R-28 = 464.1 CFU m−3) followed by the semi-basement (R-4 = 673.2 CFU m−3 and R-8 = 415.1 CFU m−3). Regarding the environmental quality, it was observed that R-4 and R-24 revealed indoor/outdoor (I/O) ratios ≥ 1.5, i.e. values of 1.5 and 1.7 respectively, indicative of a bad environmental quality, while in the remaining repositories values ≤ 1 were obtained, evidencing a good environmental quality as well as a good ventilation and aeration.
–
–
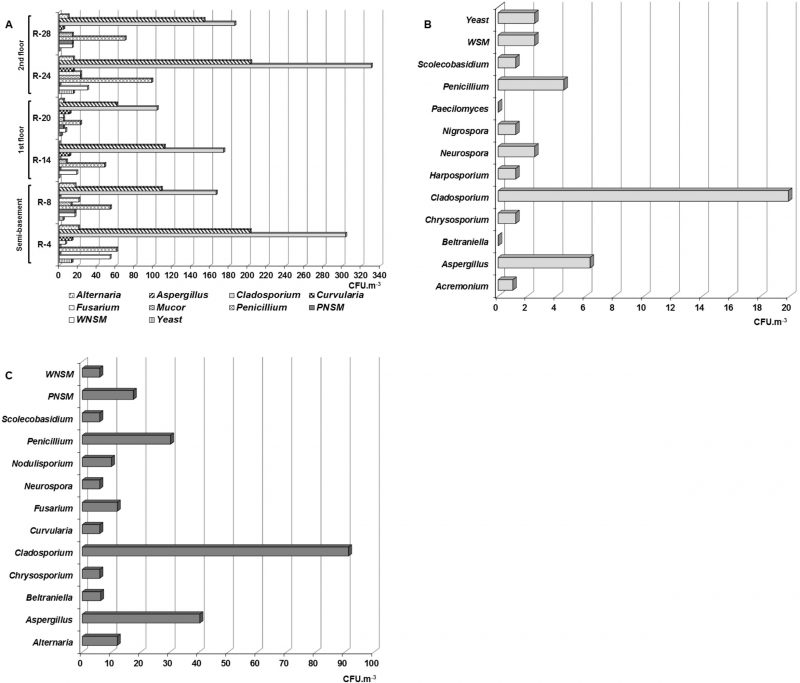
A total of seven taxa was detected in the air of the naturally ventilated repositories, predominating genera Aspergillus P. Micheli Ex Link, Cladosporium Link and Penicillium Link, which turned out to be ecologically abundant (Figure 1A). However, the genera Alternaria Nees, Curvularia Boedijn, Fusarium Link and Mucor Fresen as well as yeasts and two types of non-sporulating mycelia (WNSM: white non-sporulating and PNSM: pigmented non-sporulating) were also detected but at lower concentrations. It should be highlighted that in all the repositories located south of the building, the concentrations of these three predominant genera were higher than those obtained in the repositories located on the north side.
–
–
Although the genera Alternaria, Curvularia and Mucor, as well as the WNSM were detected in low concentrations (≤ 54 CFU m−3), they were abundant in the air of the repositories, since their relative frequency (RF) of appearance fluctuated between 83.3 and 100 %, i.e., they were detected in five of the six naturally ventilated repositories. On the other hand, it was found that the Fusarium genus and yeasts were common in these environments.
–
Eleven genera, plus non-sporulating mycelium (WNSM) and yeasts were isolated from the Map library air (Figure 1B). The genera were Acremonium Link, Aspergillus, Beltraniella Subram., Chrysosporium Corda, Cladosporium, Harposporium Lohde, Neurospora Shear & B.O. Dodge, Nigrospora Zimm., Paecilomyces Bainier, Penicillium and Scolecobasidium E.V. Abbott. Of them, Cladosporium predominated due to its concentration (19.9 CFU m−3), followed by Aspergillus (6.3 CFU m−3) and Penicillium (4.5 CFU m−3), while the other genera were detected at lower concentrations (1 – 2.5 CFU m−3).
–
From the outdoor air, eleven genera and two non-sporulating mycelia were recorded with a marked predominance of the genus Cladosporium (91.3 CFU m−3) followed by Aspergillus (40.3 CFU m−3) and Penicillium (30.3 CFU m−3; Figure 1C). The remaining genera and the non-sporulating mycelia were isolated at lower concentrations (6 – 17.5 CFU m−3).
–
Whereas the genera Aspergillus, Cladosporium and Penicillium were predominant, it should be noted that the concentrations obtained for Aspergillus and Cladosporium in most of the naturally ventilated repositories were higher than 100 CFU m−3, even in some repositories such as R-4 and R-24 the concentrations were ≥ 200 CFU m−3. However, concentrations lower than 160 CFU m−3 were obtained for the other genera.
–
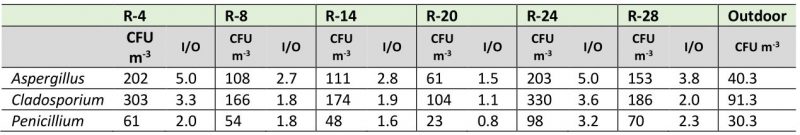
Although most of the studied environments showed typical I/O ratios of good quality environments (I/O ≤ 1), when analyzing the I/O ratio by each predominant genus, the behavior was very different. As shown in Table 2, the values of the I/O ratios for the genus Aspergillus fluctuated between 1.5 and 5, for the genus Cladosporium these values ranged between 1.1 and 3.3, and for the genus Penicillium they varied between 0.8 and 3.2. The repositories that showed the highest indexes were R-4 and R-24, while in R-14 and the Map library the ratios were less than 1.
–
TABLE 2. Concentrations and I/O ratio values for the predominant genera in each naturally ventilated repository. |
 |
–
Species belonging to the predominant genera in the air
Since genera Aspergillus, Cladosporium and Penicillium were the predominant ones, all isolated strains were identified to species level. In total, 14 species of Aspergillus, 14 Cladosporium and eleven Penicillium were identified (Table 3). Several species were abundant and within them were four Aspergillus (A. flavus Link, A. niger Tiegh., A. terreus Thom, A. versicolor (Vuill.) Tiraboschi), five Cladosporium (C. cladosporioides (Fresen.) G.A. de Vries, C. gossypiicola Pidoplichko & Deniak, C. lignicola Link, C. oxysporum Berk. & M.A. Curtis, C. sphaerospermum Penz.), and four Penicillium (P. chrysogenum Westling, P. citrinum Thom, P. commune Thom, P. simplicissimum (Oud.) Thom). A total of five species were common (A. ochraceus K. Wil., C. fulvum Cooke, C. minourae Iwatsu, C. staurophorum (Kendrick) M.B. Ellis, C. tenuissimum Cooke, Grevillea), five were frequent (A. clavatus Desm., A. flavipes (Bain & Sart) Thom & Church, A. nidulans (Eidam) G. Winter, C. herbarum (Pers. Fr.) Link, P. oxalicum Currie & Thom), three were occasional (A. oryzae (Ahlb.) Cahn, P. aurantiogriseum Dierckx, P. griseofulvum Dierckx) and ten were rare species (A. athecius (Raper & Fennell), A. glaucus Link, A. restrictus G. Smith, C. allii-porri (Saccardo & Briard) Boerema, C. caryigenum (Ellis & Lang), C. coralloides W. Yamamoto, C. elatum (Harz) Nannfeldt, P. corylophilum Dierckx, P. digitatum Saccardo, P. decumbens Thom).
–
17 species isolated from the outdoor environment were also detected in some of the indoor environments and those were A. flavus, A. niger, A. ochraceus, A. oryzae, A. terreus, C. coralloides, C. elatum, C. fulvum, C. lignicola, C. minourae, C. oxysporum, C. sphaerospermum, C. tenuissimum, P. aurantiogriseum, P. chrysogenum, P. citrinum, P. corylophilum (Table 3).
–
TABLE 3. Species of the genera Aspergillus, Cladosporium and Penicillium isolated from indoor air and dust collected in the different repositories analyzed. |
|---|
| Table 3 |
RF: Relative frequency. EC: Ecological categories [30] are Abundant taxa (A) when RF = 100 - 81%, Common taxa (C) when RF = 80 - 61%, Frequent taxa (F) when RF = 60 - 41%, Occasional taxa (O) when RF = 40 - 21% and Rare taxa (R) when RF = 20 - 0.1%. *: Indicates the existence of different percentages of teleomorphs (2-10%). 30. Borrego S, and Molina A (2014). Comportamiento de la aeromicrobiota en dos depósitos del Archivo Nacional de la República de Cuba durante 7 años de estudio. AUGMDOMUS 6:1-24. Available at: http://revistas.unlp.edu.ar/domus/issue/view/99 |
–
Characterization of the settled dust
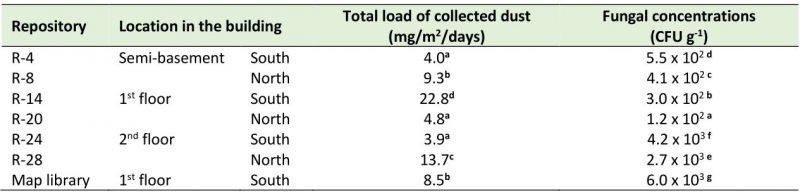
The settled dust load in the repositories showed values that ranged between 3.9 and 22.8 mg/m2/day (Table 4). It was observed that the repositories located in the semi-basement of the building (R-4 and R-8) had dust values that differed significantly, with R-8 (situated on the north side) revealing the highest accumulation of dust. The largest dust amounts were accumulated in R-14 located on the south side of the first floor and in R-24 set on the north part of the second floor. On the other hand, fungal concentrations that fluctuated between 1.2 x 102 and 6.0 x 103 CFU g−1 of dust were obtained. But the repositories set on the south side of the building showed significantly higher loads than those situated on the north part, with the highest concentrations being detected in the repositories on the second floor and in the Map library, which is a climate-controlled repository.
–
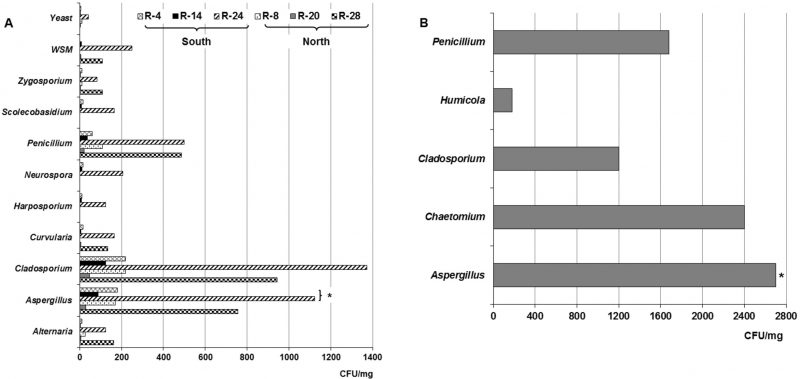
Figure 2 shows the different isolated genera from dust collected both in the naturally ventilated repositories as well as in the Map library, a climate-controlled repository. As observed in the naturally ventilated repositories, nine genera, a non-sporulating mycelium (WNSM) and yeasts were obtained, while five taxa were isolated in the Map library. The genera Alternaria, Aspergillus, Cladosporium, Penicillium and Zygosporium Mont. were isolated from all ventilated repositories. Instead the genera Curvularia, Harposporium, Neurospora and Scolecobasidium were only isolated from the dust collected in R-4, R-14 and R-24, repositories located on the south side of the building. Some Aspergillus teleomorph were detected in the dust collected only in the repositories situated in the southern part of the building (R-4, R-14, R-24 and Map library). A WNSM was isolated from the dust collected in R-14, R-20, R-24 and R-28, while yeasts were detected in five of the repositories, except in R-28. In this case again, the predominant genera were Aspergillus, Cladosporium and Penicillium. In the Map library the predominant genera were also Aspergillus (2700 CFU m−3), Cladosporium (1200 CFU m−3) and Penicillium (1680 CFU m−3), although the genera Chaetomium Kunze and Humicola Traaen were also detected at lower concentrations.
–
Species characterization in the dust
Fungal strains belonging to predominant genera Aspergillus, Cladosporium and Penicillium were identified to species level. Totally ten species belonging to Aspergillus, nine to Cladosporium and eight to Penicillium were identified (Table 3). According to the percentage of the RF, 13 species were found to be abundant and were A. chevalieri L. Mangin, A. flavus, A. glaucus, A. niger, A. restrictus, C. cladosporioides, C. herbarum, C. oxysporum, C. sphaerospermum, C. tenuissimum, P. chrysogenum, P. citrinum, P. simplicissimum. Although the species A. chevalieri, A. flavus, A. niger, C. cladosporioides, P. chrysogenum, P. citrinum and P. simplicissimum showed RF = 100%, i.e., they were detected in all repositories, their relative densities (RD) were different. In this case, C. cladosporioides showed an RD ≤ 14% in all repositories (equivalent to ≤ 17 CFU g−1 in ventilated repositories and 1200 CFU g−1 in Map library), followed by A. chevalieri, A. flavus and A. niger (RD = 3 – 17%) as well as P. chrysogenum and P. citrinum (RD = 3 – 8%), while P. simplicissimum showed a RD between 2 and 3%. In the Map library, the percentages of the three Aspergillus species mentioned above represented high concentrations (324 – 459 CFU g−1) because this genus was found at a very high concentration in the collected dust (2700 CFU g−1); the same happened with the mentioned Penicillium species, the concentrations in the dust fluctuated from 34 to 134 CFU g−1 because this genus was found at a concentration of 1680 CFU g−1. The other abundant species were detected at RF = 85.7%, i.e., in four of the five repositories (A. glaucus, A. restrictus, C. herbarum, C. oxysporum, C. sphaerospermum, C. tenuissimum), and their RD were also different but also low (equivalent to < 20 CFU g−1). The species C. sphaerospermum, C. tenuissimum and C. oxysporum presented the highest RD (RD = 3 – 10%), while A. restrictus and A. glaucus showed the lowest RD (RD = 1–3%), with the exception of A. restrictus that revealed an RD = 10% only in the dust collected in R-4. Two species were classified as common with a RF of 71.4% (A. oryzae and A. versicolor), but their RDs varied between 2 and 3%, i.e., percentages and concentrations low. Six species were frequent (A. penicillioides Spegazzini, C. basiinflatum Bensch, Crous & U. Braun, C. elatum, C. hillianum Bensch, Crous & U. Braun, P. commune and P. janczewskii KM Zalessky), but only C. basiinflatum showed a RD between 3 and 5% (equivalent to < 20 CFU g−1), the other frequent species showed smaller RD (≤ 3%), with the exception of A. penicillioides in R-14 (RD = 4%, equivalent to 13 CFU m−3) and P. janczewskii in Map library (RD = 13%, equivalent to 218 CFU g−1). Five species were classified as occasional (A. clavatus, A. terreus, P. aurantiogriseum, P. decumbens and P. digitatum) and all showed a low RD (≤ 3%). Only one species was found to be rare (C. caryigenum) and was isolated from the Map library with an RD of 6%, equivalent to 72 CFU g−1.
–
–
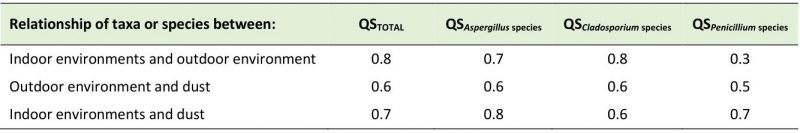
Similarity of the different taxa obtained in air and dust
Comparisons were made between the three niches studied (indoor air, outdoor air, and dust collected indoors). Taxa isolated from indoor air were compared with those obtained from outdoor air. Taxa from indoor air were compared with those in collected dust. Taxa detected in outdoor air were likened with those in collected dust (Table 5). When the isolated taxa from the indoor environment were compared with those detected in the outdoor air a very high Sørensen's coefficient of similarity (QS) was obtained (QS of 0.8). In that case, nine taxa were similar between both environments and they were Alternaria, Aspergillus, Chrysosporium, Cladosporium, Curvularia, Fusarium, Neurospora, Penicillium and Scolecobasidium. The QS of Aspergillus, Cladosporium and Penicillium species between these two environments were 0.7, 0.8 and 0.3, respectively, showing that the greatest similarities were between Cladosporium and Aspergillus. The species found both indoors and outdoors were C. cladosporioides, C. oxysporum, C. tenuissimum, C. sphaerospermum, C. elatum, C. fulvum, C. lignicola, C. minourae, A. flavus, A. niger, A. ochraceus, A. oryzae, A. parasiticus, A. penicillioides and A. terreus.
–
The isolated taxa in the indoor air and dust showed a QS of 0.7, revealing a high similarity. Eight taxa were found to be similar between both niches and they were Alter-naria, Aspergillus, Cladosporium, Curvularia, Harposporium, Neurospora, Penicillium and Scolecobasidium. Regarding Aspergillus, Cladosporium and Penicillium species, the QS obtained were 0.8, 0.6 and 0.7, respectively. The commoly isolated species were A. clavatus, A. flavus, A. glaucus, A. niger, A. oryzae, A. penicillioides, A. restrictus, A. terreus, A. versicolor, C. caryigenum, C. cladosporioides, C. elatum, C. herbarum, C. oxysporum, C. sphaerospermum, C. tenuissimum, P. aurantiogriseum, P. chrysogenum, P. citrinum, P. commune, P. decumbens, P. digitatum and P. simplicissimum.
–
Taxa similarity between the outdoor environment and dust yielded a QS of 0.6; this coefficient shows a moderate similarity with seven similar genera (Alternaria, Aspergillus, Cladosporium, Curvularia, Neurospora, Penicillium and Scolecobasidium). However, when comparing the taxa isolated in these two niches with those detected in the indoor air of the repositories, it is noted that there was a similarity in seven of them too (Alternaria, Aspergillus, Cladosporium, Curvularia, Neurospora, Penicillium, Scolecobasidium). QS obtained for Aspergillus, Cladosporium and Penicillium species between outdoor air and dust were 0.6, 0.6 and 0.5, respectively, showing a moderate similarity. Aspergillus flavus, A. niger, A. oryzae, A. penicillioides, A. terreus, C. cladosporioides, C. oxysporum, C. tenuissimum, C. sphaerospermum, C. elatum, P. aurantiogriseum, P. citrinum and P. chrysogenum were found in indoor dust and in outdoor air. When comparing these species with those obtained in the indoor air of the repositories, it can be seen that all of them are similar.
–
–
Characterization of the biodeteriogenic potential
The biodegradative activities were only determined for the predominant genera species (Aspergillus, Cladosporium and Penicillium) that were isolated from indoor air and dust in the studied repositories (Table 6). Of them, ten strains isolated from air (27%) showed high CEI values (IE ≥ 0.7), but only A. niger 1, A. flavus 1 and P. chrysogenum 1 exhibited the highest IE (> 0.75). The rest of the strains had the following behavior: 14 strains (37.8%) exhibited a moderate CEI and twelve strains (32.4%) showed a low CEI; only C. oxysporum 1 was not able to degrade cellulose. It is necessary to note that although 75% of the Aspergillus strains exhibited a moderate to high activity, the Penicillium strains revealed a higher ability (90.9%).
–
Regarding the amylolytic activity, the majority of the strains (78.4%) showed a moderate or low activity; only four strains evidenced a high activity (A. flavus 1, A. niger 1, A. ochraceus 1, C. herbarum 1) and other four did not degrade the starch. Likewise, 59.5% of the strains degraded gelatin moderately or with a low EI (PEI ≤ 0.69) and only nine strains (24.3%) showed the highest PEI (A. flavus 1, A. glaucus 1, A. niger 1, A. nidulans, A. ochraceus 1, A. oryzae 1, C. oxysporum 1, P. chrysogenum 1, P. oxalicum) while six strains (16.2%) did not showed activity. Although acids were excreted by all the taxa, ten of them (27%) should be highlighted for having decreased the pH of the culture medium to values below 5 (A. flavipes, A. nidulans, A. oryzae 1, A. terreus 1, A. versicolor 1, C. cladosporioides 1, P. brevicompactum, P. chrysogenum 1, P. corylophilum, P. oxalicum); whereas, 18 strains excreted different pigments (48.6%) with the prevalence of yellow, green dark and brown colors.
–
Also, all the species isolated from the dust were able to degrade cellulose with different intensity. Eight strains revealed very high enzyme indices (EI ≥ 0.7), but among them two strains stood out (A. niger 2, A. oryzae 2) since their CEI were the highest (EI > 0.75).
–
–
In relation to the number of strains detected on indoor air by genus with moderate or high CEI, it was obtained that Penicillium showed ten strains (27%), followed by Aspergillus (nine strains, 24.3%) and Cladosporium (5 strains, 13.5%). However, the strains detected in the dust had a different behavior, since Aspergillus showed the highest strains number (seven) with a moderate or high CEI (25.9%) followed by Penicillium (five strains, 18.5%) and Cladosporium (four strains, 14.8%).
–
Of the total strains detected in the dust, 29.6% were able to express the highest amylolytic indexes (AEI ≥ 0.7), while the 25.9% of them exhibited a moderate AEI with a slight prevalence of Cladosporium species (11.1% of Cladosporium species vs. 7.4% of Aspergillus and Penicillium species); but one strain did not show amylolytic activity (P. aurantiogriseum 2). In relation to the proteolytic activity, 18 strains (66.7%) showed a moderate or high PEI, highlighting four of them (A. niger 2, C. basiinflatum, P. decumbens 2, P. simplicissimum 2) while only one strain did not degrade proteins (P. commune 2). Although all strains excreted acids, eight of them (29.6%) caused a marked decrease in pH (pH < 5); therefore, they were the biggest producers of acids while 15 strains (55.6%) excreted pigments with a prevalence of yellow and brown colors.
–
As can be seen in Table 6, of indoor air, twelve strains (32.4%) showed positive results in the five tests performed (cellulolytic, amylolytic, and proteolytic activity, as well as excretion of acids and pigments), 21 of them (56.8%) were positive in four of the five tests, three strains (8.1%) were positive in three of the tests, while only one strain (2.7%) showed positivity in two of the analyzes performed. Regarding the detected strains in the dust, it was obtained that 14 of them (51.9%) gave positive results to the five tests, twelve strains (44.4%) were positive in four analyzes and only one strain (3.7%) was positive in three assays. It should be noted that of the isolated strains from both indoor air and dust, it was obtained that nine of them (three from air and six from dust) showed the highest risk for the preserved documents, since they displayed the greatest degrading ability (A. flavus 1, A. niger 1, A. ochraceus 1, A. niger 2, A. oryzae 2, C. basiinflatum, P. citrinum 2, P. decumbens 2, P. simplicissimum 2).
–
TABLE 6. Enzyme Index (EI) obtained of Aspergillus, Cladosporium and Penicillium strains isolated from the indoor air and collected dust from NARC repositories. |
| Table 6 |
CEI: Cellulolytic Enzymatic Index. AEI: Amylolytic Enzymatic Index. PEI: Proteolytic Enzymatic Index. Enzymatic index (EI) = 0.5 - 0.59 is low, EI = 0.6 - 0.69 is moderate, EI ≥ 0.7 is high. +: Indicates excretion of pigments. -: Indicates no excretion of pigment. pH values < 7 reveal acid production. *: These pigments were detected in CMC medium and a culture medium with similar composition to CMC but with glucose (1%) as control. |
DISCUSSION
Studies of airborne fungi in indoor environments always reveal the taxa diversity in the air. But airborne microorganisms, particularly fungi, do not remain suspended in the air indefinitely. They settle at some time and, under favorable conditions, may grow on the surfaces where they have settled. Therefore, airborne fungi are a potential risk factor for biodeterioration [3][11]. The same happens with dust, which, when settling on objects and documents, and under favorable conditions of temperature (T) and water activity (aw), can favor the growth of fungi [6][11].
–
For years, NARC has performed fungal concentration determinations in the indoor environments of their repositories [30][31][33][34][36]. But many of these studies were made using a sedimentation method whose results are not comparable because as it is not quantitative. However, in other investigations where a biocollector was used, the reported total concentrations of fungi were similar to those obtained in this study. Borrego and Perdomo [29] carried out a study in six repositories with natural cross-ventilation systems and reported fungal concentrations that ranged between 60 and 550 CFU m−3. Later in a similar study [32] the referred concentrations ranged between 92 CFU m−3 and 785 CFU m−3. Likewise, these data were similar and/or lower than those obtained by other authors [28][37]. On the other hand, previous studies performed in the Map library of NARC report concentrations of 38 CFU m−3 [20] and 40.8 CFU m−3 [26].
–
All these total concentrations of fungi in air showed a tendency to be higher in the repositories located in the semi-basement and the second floor of the building and to be lower in those repositories located on the first floor. This pattern was similar to a previous report [32]. If the indoor environment is influenced by the outdoors [4][14][32][38][39][40], then it would be expected that the concentration should decrease with the increase in height, a matter that did not occur. This was possibly due to the fact that the building, due to its location within the city, has been constantly influenced by high pollution [41] and a high movement of bioaerosols. This may be due to the interrelation of two fundamental aspects. The first is justified by the size of the fungal spores. Fungi with small spores were detected in the outdoor environment of the archive building (e.g., Aspergillus, Penicillium, Cladosporium, Chrysosporium, etc.), and it is known that smaller spores are dominant at higher altitudes, while larger spores and conidia are more frequent at lower levels [42][43]. The second is associated with the high vehicular and pedestrian traffic that occurs in the streets surrounding the archive, which together with the wind generate turbulence of dust and bioaerosols (harbouring small fungal spores) that impact the building, facilitating their entry to the repositories through the ventilation ducts. This phenomenon is favored by the large number of gardens with trees in the surroundings of the building [4].
–
When the obtained fungal concentrations were compared with the threshold limits reported by Roussel et al. [16], it was evidenced that R-4 and R-24 had environments with high pollution since the concentrations were between 560 and 1000 CFU m−3, while the rest of the repositories showed environments with low to moderate pollution. This behavior coincides with the limit established by the Portuguese legislation of 500 CFU m−3 for fungi [18], when the results were compared.
–
Nevertheless, it is suggested that the outdoor airborne fungi concentration is generally higher than that in the indoor environment, as the outdoor environment is a significant modulator of fungal concentrations in naturally ventilated indoor environments [11][28]; hence, it has been reported that the I/O ratio is an indicator of microorganism emission. If this ratio is ≤ 1, the outdoor environment is the main source of bioparticle emission into the indoor environments [11][44], but if the I/O ratio is higher than 1 then there are indoor sources of contamination [11][28][44]. According to these criteria, variations in the pollution were observed. In R-4 and R-24 the calculated I/O ratios revealed values of 1.5 and 1.7, typical of bad environmental quality, while the rest of the repositories presented I/O ratio values lower than 1, which shows good environmental quality. It is possible that in R-4, because it is located in the semi-basement, there is a lack of air circulation and this caused conditions that favored the appearance of amplification areas, stimulating an increase in the fungal concentration inside. But in the case of R-24 it is possible that two aspects are taking place at the same time. The first is related to the ducts that are located in about 4.5 m height. As these ducts are rarely cleaned, it is very likely that they are contributing to the continuous introduction of fungal propagules into the repository air; while the second aspect is linked to the bioparticles that remain attached to the repository walls which are not very smooth. It is possible that these particles, together with the T and relative humidity (RH) conditions existing in the repository, are causing the fungal growth not visible to the naked eye in some areas of the walls, when they are impacted by the air they release propagules to the indoor, increasing the environmental fungal load and, hence, the I/O ratio. About the documentation preserved in both repositories, in systematic reviews made by the conservators and researchers, fungal growth has not been detected in them. Therefore, apparently it is not the documents that are contributing to the high I/O ratio. However, it is important to perform systematic fungal isolations from the documents to prove this hypothesis.
–
From the indoor air of the naturally ventilated repositories, a total of seven genera, two non-sporulating mycelia and yeasts were isolated, while from Map library eleven genera, a non-sporulating mycelium and yeasts were detected, with a marked prevalence of the genera Aspergillus, Cladosporium and Penicillium in all of them. Similar results were obtained in previous studies carried out at the NARC [20][26][29][30][32][33][34] and in other Cuban archives and museums [45][46][47][48][49][50][51]. These genera are also very common in the NARC outdoor air [52]. However, Acremonium and Beltraniella that were first isolated in the Map library environment showed a low ecological impact, turning out to be rare genera. In some previous studies performed in NARC, in other Cuban archives, as well as in archives, library and museums of other countries, findings of non-sporulating mycelia have been reported, referring mainly to two of them, one that is white to the naked eye (WNSM) and the other that is pigmented (PNSM) [20][26][44][45][46][53]. These mycelia have been detected in different concentrations and are still being isolated from the environments of the NARC repositories as shown in this study.
–
When comparing the taxa obtained in the indoor air with those the outdoor one, it was observed that ten taxa were detected in these environments for a 66.7% of coincidence. The matching genera in both environments were Alternaria, Aspergillus, Beltraniella, Chrysosporium, Cladosporium, Curvularia, Fusarium, Neurospora, Penicillium and Scolecobasidium. It should be noted that most of these taxa have been reported by other authors in archives, libraries and museums in various countries [28][37][38][48][54]. These results show that there is a high degree of coincidence in the fungal diversity between the indoor and outdoor environments, evidencing the high influence of the outdoor environment on the mycobiota of indoors, an aspect attributable to the existence of natural ventilation in most of the repositories.
–
Regarding the I/O ratios of the predominant genera Aspergillus, Cladosporium and Penicillium, high values were obtained in most of the repositories (1.8 – 5.0), suggesting that several problems are occurring: a constant introduction of dust into the repository air, the existence of amplification zones, and the presence of fungal growth on the repository walls and perhaps on other substrates not yet identified by us, the NARC conservators and researchers. A similar behavior for the genera Aspergillus and Penicillium was reported by Pyrri et al. [28].
–
Several species were isolated from the indoor air, which turned out to be ecologically abundant. They were A. flavus, A. niger, A. terreus, A. versicolor, C. cladosporioides, C. gossypiicola, C. lignicola, C. oxysporum, C. sphaerospermum, P. chrysogenum, P. citrinum, P. commune and P. simplicissimum; although other species of Aspergillus, Cladosporium and Penicillium were also detected with minor RF. It is noteworthy that the species Cladosporium allii-porri, C. elatum, C. fulvum, C. minourae and P. corylophilum were new findings for Cuban archives environments.
–
About the settled dust in the studied repositories, it should be noted that the values obtained in this research were higher than those reported by Awad et al. [11] in a study carried out in a museum in Cairo, Egypt, where they registered loads of 0.1-8.4 mg/m2/day in the different analyzed spaces, but in the repositories the dust burden was very low (0.1-1.2 mg/m2/day). However, the obtained values were much lower than those reported by Rodríguez [48] in a study carried out in the repository of the National Museum of Music in Cuba (64 mg/m2/day) a heritage institution located in Old Havana, very near the Port Avenue and the NARC. On the other hand, the fungal load values were also lower than those reported by Valdés et al. [55] in an investigation performed inside the Basilica of the San Francisco de Asís Convent (26.5 – 52.8 mg/m2/day), an institution that is also located very close to Port Avenue and the NARC and, consequently, it can be stated that the amount of dust collected from these repositories was low. In the statistical analysis carried out to compare the accumulated dust load between the two sites of the building (north and south), significant differences were found between the repositories on the first floor and the rest of the repositories, being R-14 the one that showed the highest values. This repository is one of the most exposed to the entry of dust through the ventilation ducts, since it is located on the south side of the building, the part that receives the greatest impact from outside pollution [40] and dust due to the existence of large avenues, groves, high vehicular and pedestrian traffic, etc. Although the dust accumulation within a built environment is the result of many factors (open/closed windows, ventilation system, human activities, green areas around the property, etc.) and not only the influence of the outdoor, in this study a marked influence was evidenced from the outdoor in the settled dust load, in agreement with previous reports [4][13][24][56]. This may be due to the constant entry of dust through the natural ventilation system. Although a good part of the dust is trapped in the ventilation ducts, it is not always systematically removed from all of them. The ducts located at a height of 1.5 m are vacuumed once a month at the same time as the outside of the documents, the shelves and the floor, but those located at 4.5 m height are rarely cleaned, hence they represent important elements of the continuous entry of dust into the repositories.
–
In this study the fungal loads that were detected in dust were kept in lower ranges and/or similar to those reported by other authors. The fungal burden associated to the settled dust on selected books or archive materials in Polish libraries and one archive oscillated between 49 x 103 – 450 x 103 CFU g−1 of dust [14], while the fungal concentration obtained from the surfaces of books, shelves and furniture of two Egyptian libraries ranged between 0 – 6.3 x 105 CFU g−1 [17]. However, fungal concentrations associated with deposited dust in an Egyptian museum ranged from ∼ 102 to 104 CFU g−1 [11]. The fungal concentrations detected in this study were markedly lower than those reported by OSHA [57], which establishes limit concentrations in the order of 106 CFU g−1 of dust. It is known that many microorganisms are associated with dust [58][59] and in archives and libraries where dust tends to be high, this phenomenon is more marked [4][6][14]. Therefore, the sanitation strategy of the repositories should be improved to ensure not only their cleanliness but also a convenient way to cleanse the ducts located at 4.5 m height. Frequent cleansing removes the accumulated dust, prevents the resuspension of all settled allergens, thus eliminating one of the basic sources of indoor fungal aerosol. These measures are part of the strategies that guarantee the preservation of the documentary collections in the archives.
–
In the collected dust, 14 taxa were detected, some of them such as Alternaria, Aspergillus, Cladosporium, Penicillium and Zygosporium were isolated from the dust, of the all naturally ventilated repositories, other were only detected in the dust collected in the repositories located on the south side of the building (R-4, R-14 and R-24) e.g. Curvularia, Harposporium, Neurospora and Scolecobasidium. Yeasts were detected in almost all repositories except in R-28 (located on the 2nd floor). It should be noted that Aspergillus teleomorphs were detected only in the collected dust of repositories located in the southern part of the building. This may be due to the characteristics of the dust (water activity, pH, etc.) in that area, which could have guaranteed the viability and subsequent development of these teleomorph. Aspergillus teleomorphs in indoor dust has also been detected in previous studies [6][16][17].
–
In this case, Aspergillus, Cladosporium and Penicillium were again predominant, although Alternaria and Zygosporium also prevailed. These genera abound in the atmosphere of Havana and have been previously detected, although Curvularia and Alternaria are also among the most common genera in this environment [51][60] and may have accumulated in the dust that penetrated the repositories and settled in the collectors.
–
Aspergillus, Cladosporium and Penicillium were previously detected as predominant genera in the dust of an Italian archive [6], in French archives [16] and an Egyptian museum [11]. In investigations made in Polish archives, libraries and museums, several species of these three genera were isolated among dust fungi by a method different from that used in this study [14][48][56][61]. On the other hand, these genera were also predominant in household dust [7], although their characteristics may be different from those of an archive, library or museum.
–
The presence of Harposporium, Neurospora and Scolecobasidium genera in the collected dust of the ventilated repositories located to the south of the building, as well as Humicola genus detected only in the Map library dust (also located at south side) may have originated from the vegetation found in the adjacent park and in an area closely situated in this side of the building [62][63][64]. Fungal diversity in the dust could be influenced by the green areas and the total area of vegetation in the vicinity of the studied site [4].
–
About the species belonging to the predominant genera in the dust (Aspergillus, Cladosporium and Penicillium), it was shown that 48.1% of them were abundant according to their percentages of RF; the remaining species were classified as common (7.5%), frequent (22.2%), occasional (18.5%) and rare (3.7%). The distribution of the abundant species by genus showed that 50% of them corresponded to the Aspergillus genus, distinguishing A. chevalieri, A. flavus and A. niger, and 55.6% belonged to Cladosporium, standing out C. cladosporioides and C. sphaerospermum. It was also found that 37.5% of Penicillium species were abundant, highlighting P. chrysogenum and P. citrinum.
–
In this study, for the first time, the similarity (QS) of isolated taxa in the three analyzed niches (indoor air of the NARC repositories, outdoor air and settled dust) was analyzed. High QSs were obtained by comparing taxa detected in indoor air and outdoor air as well as between indoor air and settled dust. Hence, the contribution of the species belonging to the prevailing genera depended on the analyzed niche. The comparison of species between indoor and outdoor air showed a high QS for Aspergillus and Cladosporium, while for Penicillium it was low since species of this genus were only detected in the first sampling. However, the QSs obtained when comparing indoor air and dust were high (QS ≥ 0.6), particularly for the Aspergillus genus (QS = 0.8). These results showed that the outdoor air has an important influence on the repository's indoor air, mainly due to the existing natural cross-ventilation system and that the dust particles within the repositories (either because they penetrated from the outdoor or because they come from the ventilation ducts, or from walls with fungal growth, etc.) contribute significantly to the enrichment of airborne mycobiota indoors. Twelve of the detected species in this study were similar in the three analyzed niches and were A. flavus, A. niger, A. oryzae, A. penicillioides, A. terreus, C. elatum, C. oxysporum, C. sphaerospermum, C. tenuissimum, P. aurantiogriseum, P. chrysogenum and P. citrinum. Some of these species are recognized for their allergenic properties [22], while other species isolated in this work (e.g. A. chevalieri, A. clavatus, A. flavipes, A. glaucus, P. brevicompactum, P. commune, etc.) are considered opportunistic pathogens [65].
–
Many of the species detected both in the repositories indoor air and in the settled dust have been previously isolated from the outdoor air [52][60][66], suggesting that they are typical species of the Havana environment, although they have also been reported in countries of other world tropical and subtropical regions [42] characterized by high T and RH values, mainly the species of the genus Aspergillus [60][67][68]. It has even been reported that Aspergillus and Cladosporium correlate positively with high T and RH values [60][69][70], and that in particular RH contributes to spore dispersion and to maintain a high viability of its propagules in the air [60][69].
–
On the other hand, in this study species belonging mainly to primary and secondary colonizers were also detected [11][71], both in the repositories air and in the settled dust, demonstrating the risk that documentary collections run of being damaged by these fungi. In addition, poor air circulation may facilitate the deposition of airborne fungal propagules on documents, thus facilitating fungal growth on these materials. For this reason, some degradative tests were carried out on the isolated strains with the intention of learning their biodeteriogenic potential.
–
As it was shown, all strains isolated both in the air and in the settled dust were able to degrade to a greater or lesser extent the tested nutritional sources that are part of the components of paper and other documentary materials (cellulose, starch, gelatin), it is important to pay special attention to the preventive conservation measures fundamentally related to the hygiene of documents and repositories, with the guarantee that all documents have quality packaging that mitigates the harmful effect of dust and environmental fungi, as well as a good air circulation in the repositories since the T and RH cannot be controlled due to the existing natural ventilation system in these premises. Fungi are mostly mesophilic, acidophilus (pH 4 – 6) and grow well at relative humidity above 70%. Only if the temperature, water activity and acidity in the substrate are favorable, the fungal spores settled on the documents can germinate and grow abundantly; but the main limiting factor that determines the development of fungi in these materials is water, although some xerophilic/halophilic fungi have been associated with these materials [72]. It has been reported that species of Aspergillus (e.g., A. flavus, A. niger, A. terreus), Cladosporium (such as C. cladosporioides, C. sphaerospermum) and Penicillium (e.g., P. chrysogenum and P. citrinum) have cellulolytic, amylolytic and proteolytic activities, excrete acids and many of them also excrete pigments [17][19][20][35][47][48][51]; hence, they are species that have been linked with the documents' biodegradation [21][25][38]. Specifically, the aforementioned species and many others were capable of degrading cellulose, starch and gelatin. Among them, the following were distinguished by their high CEIs: A. chevalieri, A. clavatus, A. ochraceus, A. oryzae, A. versicolor, C. basiinflatum, C. caryigenum, P. decumbens, P. griseofulvum and P. simplicissimum. The species A. ochraceus, A. oryzae, A. versicolor, C. herbarum, C. basiinflatum and P. simplicissimum outstand for their high AEIs, while A. glaucus, A. ochraceus, A. oryzae, C. basiinflatum, C. cladosporioides, C. oxysporum, P. decumbens, P. digitatum, P. oxalicum and P. simplicissimum stood out for their high PEIs. But the most important aspect in the document's preservation is the number of degradative attributes that one fungal strain has [20] because it is indicative of its biodeteriogenic potential. However, it was shown in this investigation that most of the evaluated strains were positive to four or five of the analyzed physiological tests, showing that they are high-risk agents for the documentary collections preserved at the NARC. Similar results were previously reported [20][26][50].
–
The obtained results show the potential risk to which the documents conserved in these repositories are exposed. Hence, the importance of maintaining a systematic prevention strategy in the NARC where the hygiene-sanitary conditions of the repositories, furniture and documents are the most important aspect to take into account, hygiene actions that must always be carried out include the usage of appropriate personal protective equipment. The NARC has had a Preventive Conservation Plan for years that systematically executes and updates what has allowed it to preserve the documentary heritage it treasures in good condition despite the climatic conditions of the country. Just all of the research being done at NARC on airborne fungi, environmental mycological quality, and other environmental aspects contributes to the continual improvement of that Plan.
MATERIALS AND METHODS
Characteristics of the analyzed repositories
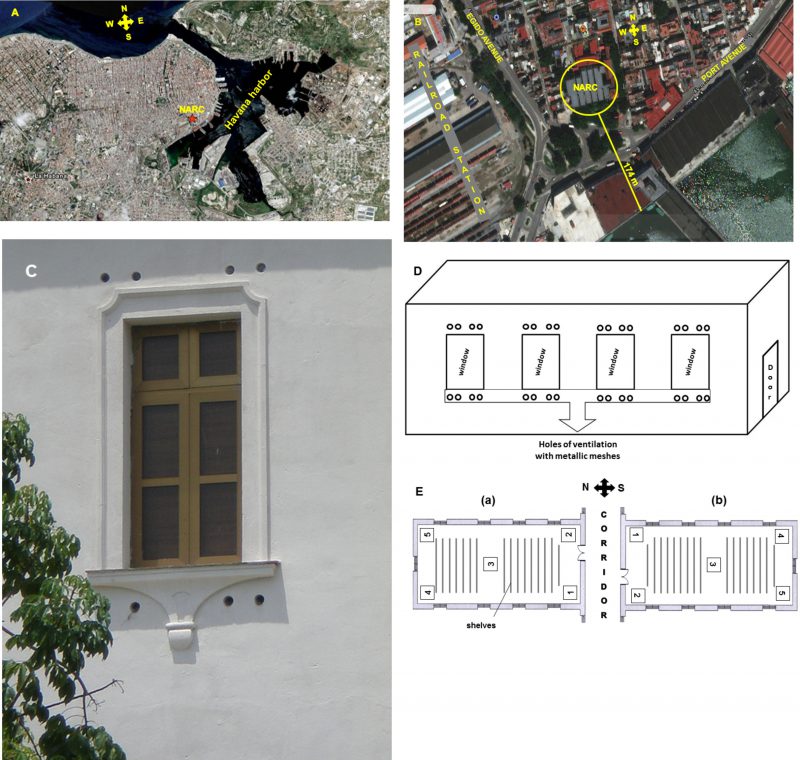
The building of the NARC was constructed in the period of 1940 to 1944 in an old urban area of Havana city known as Old Havana, 174 m from Havana harbor (Figure 3A and 3B). The NARC building is located in a highly polluted area of Havana [41]. Bordering its southern part there is a park with a lot of vegetation and a wide avenue (Port Avenue) that has a high pedestrian and vehicular traffic that generates a lot of environmental dust. Also, on its north, east and west sides it has a large number of houses that generate garbage and dirt that is deposited in garbage tanks until it is collected. In addition, it is very close to the Havana harbor, an electricity plant, the railroad station and other busy avenues.
–
The building has 30 repositories located on three floors. The majority of the repositories (28) have holes (10 cm diameter) that cross over the outer walls in an angle of 45° approximately, and they are protected by metallic meshes. These holes were made at different heights (1.5 and 4.5 m, approximately) to facilitate the inflow of outside air into the repositories and to secure a good natural cross-ventilation system (Figure 3C and 3D). Also, NARC has two repositories with air-conditioning and one of them is the Map library.
–
For the selection of the repositories with natural cross ventilation, their distribution on the three floors of the building and their location on the north or south side were considered. Of the two air-conditioned repositories, the Map library was selected because it is the largest (T = 22 ± 2°C y RH = 50 ± 5%). The studied repositories were R-4 and R-8 (situated on the semi-basement), R-14 and R-20 (in the first floor), R-24 and R-28 (in the second floor), as well as the Map library (in the first floor, too). These repositories are characterized by their large size, their dimensions (length x width x height, m) being the following: R-4: 15.2 x 6.2 x 2.5; R-14, R-24 and Map library: 15.2 x 6.2 x 5; R-8: 25.3 x 6.2 x 2.5; R-14 and R-28: 25.3 x 6.2 x 5.
–
Airborne fungal sampling
The number of points to be sampled was determined according to Sanchis [73] who reports a simple method based on the cube root of the volume of the premises (Figure 3E). According to this criterion, five points were sampled in triplicates.
–
In the environmental microbiological samplings of indoor and outdoor points a SAS biocollector (Super 100 TM, Italy) was used with an air flow of 100 L min−1 for 2 min at a height of 1.5 m in vertical position at intervals of one hour between replicates. Two variants of the culture medium were used to guarantee the greatest possible fungal diversity and were Malt Extract Agar (MEA; Biocen, Cuba) at pH 5 [52] and MEA supplemented with NaCl (7.5%) [26][32]. Then, the dishes were invert incubated for 5 to 7 days at 30°C. After the incubation, colony forming units were counted, which were them transformed into colony forming units per m3 (CFU m−3) following the instructions described in the equipment manual [74].
–
The indoor/outdoor (I/O) ratio was calculated according to Stryjakowska-Sekulska et al. [44], Awad et al. [11] and Karbowska-Berent et al. [14]. These authors reported that a good quality environment has an I/O ratio ≤ 1, and that an I/O ratio > 1 is a very strong indication of indoor sources of contamination.
–
–
Settled dust and its fungal load
A passive method was used in sample the dry settled dust. For this, six months before the environmental microbiological sampling, dust collectors were located in the same five points where the environmental sampling was carried out. As these collectors were placed on the shelf, they were at a height of 3 m from the floor in the repositories with natural cross ventilation and at 2 m in the Map library. These collectors consisted of sterile 110 mm plastic Petri dishes (previously weighed) that were left open for six months after being placed at the sampling points. Thus, the dust was deposited on the surface of the open dishes in the same way that it does on the documents [75]. During the capture of the dust collectors, they were closed with their corresponding covers and were transported to the laboratory. Subsequently, they were placed in desiccators with silica gel and weighed every 24 h until the collectors reached constant weight.
–
The determination of the total load of settled dust was carried out according to the formula proposed by Oliva et al. [76], and the deposition rate was calculated as mg/m2/day.
–
Total dust load = (Pf – Pi) / A x t
–
Where: Pf- final dry weight of the Petri dish with powder, Pi- initial dry weight of the sterile Petri dish, A- area of the dish (m2), t- time (days)
–
For the microbiological sampling of the dust, 0.01 g of the settled dust was taken from each collector and 0.5 mL of sterile distilled water was added. Samples were allowed to stand for 1 h and each sample was randomly shaken well at intervals for 45 min. Then serial dilutions were made and seeded in depth in 110 mm plates with MEA + NaCl and MEA at pH 5. Dishes were invert incubated for 5 to 7 days at 30°C. After the incubation, the colony forming units were counted in order to determine the fungal concentration expressed in colony forming units per g of collected dust (CFU g−1).
–
CFU g−1 = (Number of total CFUs obtained x dilution) / 0.01 g of collected dust
–
Identification of airborne and dustborne fungi
For the identification of fungal isolates cultural and morphological characteristics of the colonies, as well as the conidiophores and conidia structures were observed in a stereomicroscope (X14) and a clear field trinocular microscope (Olympus, Japan) at X40 and X100 coupled to a digital camera (Samsung, Korea). Different mycological key manuals were used [62][77][78][79][80][81][82][83][84][85].
–
Ecological approaches
Relative Density (RD) of fungal genera isolated from indoor air of each repository was conducted according to Smith [86], where:
–
RD = (number of colonies of one taxon / total number of colonies) x 100
–
Relative Frequency (RF) of the fungal species detected on indoor environments as well as the genera and species isolated from dust collected was determined according to Esquivel et al. [87], where:
–
RF = (times a genus is detected / total number of sampling realized) x 100
–
The ecological categories are classified as: Abundant (A) with RF = 100-81%, Common (C) with RF = 80-61%, Frequent (F) with RF = 60-41%, Occasional (O) with RF = 40-21%, Rare (R) with RF = 20-0% [32].
–
The Sørensen's coefficient of similarity (QS) was used to compare the similarities of obtained taxa among the three ecological niches (indoor air, outdoor air, and collected dust). The comparisons made were between the indoor air and the outdoor as well as between the indoor air and the collected dust [52].
–
QS = 2a/b + c
–
Where: a- is the number of common genera detected in the two environments that are comparing, b- the number of detected taxa only in indoor environment and c- the number of detected taxa only in the outdoor environment.
–
The QS values must range between 0 and 1. A value equal to 0 indicates that the taxa obtained in both compared environments are completely different and a value equal to 1 display that the taxa are identical [88].
–
Determination semi-quantitative of the biodegradation potential of the isolated taxa
Determination of enzymatic index (EI)
To quantify the cellulolytic, amylolytic and proteolytic enzymatic index (EI), the following formula was used [26][89][90]:
–
EI = 1- Dc / Dca
–
Where: Dc is the colony diameter, Dca is the sum of Dc and the diameter of the hydrolysis zone. Values between 0.5 and 0.59 were classified as low EI, between 0.6 and 0.69 as moderate EI, and above 0.7 as high. Each determination was made in triplicate and averages are reported.
–
Cellulolytic enzymatic index (CEI)
The different strains of each species were inoculated in Petri dishes containing an agarized culture medium with a saline composition for one liter of: sodium nitrate 2 g, potassium phosphate 1 g, magnesium sulfate 0.5 g, ferrous sulfate 0.01 g, chloride potassium 0.5 g, yeast extract 0.5 g and 20 g of agar technical No. 1. As a carbon source, carboxymethylcellulose (CMC) at 1% was added and incubated at 30°C. After seven days, a solution of Congo red (0.05 g l−1) was added to each dish and was maintained by one hour, then the solution was decanted and NaCl at 1 mol l−1 was added for 10 min. Cellulolytic activity was evidenced by the formation of a white halo around the colony [26].
–
Amylolytic enzymatic index (AEI)
Each strain was inoculated in a Petri dish with a saline composition similar to the one previously used and starch was (1%) employed as the carbon source. After 7 days of incubation at 30°C, approximately 5 ml of Lugol's reagent were added to each culture dish, and the presence of a colorless zone around the colonies was taken as positive hydrolysis [20][24][26].
–
Proteolytic enzymatic index (PEI)
The strains were inoculated in dishes containing an agarized culture medium with a saline composition similar to that used previously, with gelatin as the carbon source (1%). The dishes were incubated at 30°C; the test reading was performed at 7 days of incubation with the addition of the Frazier reagent. A white precipitate around the colony (halo) was taken as the presence of non-hydrolyzed gelatin but a colorless halo indicated gelatin hydrolysis [26][90][91].
–
Determination of the acid excretion
A suspension of spores from each strain was seeded (0.1 ml) in a minimal liquid medium of identical composition to the one above, but with glucose at 1% as carbon source, 0.03% of phenol red and the pH adjusted to 7. The cultures were incubated at 30°C for 3 days. A change of color from red to yellow was indicative of the production of acids and the pH of the culture medium was measured using a pH meter (Pacitronic MV 870, USA) [20][26].
–
Determination of extracellular pigments excretion
The strains were inoculated in tubes with slants containing an agarized culture medium with a saline composition similar to CMC medium but with glucose as the carbon source (1%). The tubes were incubated at 30°C during 7 days and excretion of diffusible pigments was observed in the culture medium of each tube. Also, the pigment excretion in the medium with CMC was taken into account [26].
–
Statistical analysis
In the statistical processing of the data the Statgraphics Centurion XV program was used. Student's t test was used to compare the obtained values in the repositories located to the south and the north in the same floor of the building. A simple variance analysis (ANOVA-1) and Multiple Ranges test by the method of least square difference (LSD) were performed to compare the obtained data of the total dust loads and fungal concentrations. A p-value smaller or equal to 0.05 was considered statistically significant.
REFERENCES
- Fröhlich-Nowoisky J, Kampf CJ, Weber B, Huffman JA, Pöhlker C, Andreae MO, Lang-Yona N, Burrows SM, Gunthe SS, Elbert W, Su H, Hoor P, Thines E, Hoffmann T, Després VR, and Pöschl U (2016). Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos Res 182: 346-376. 10.1016/j.atmosres.2016.07.018
- De Nuntiis P, and Palla F (2017). Bioaerosols. In: Palla F, Barresi G, editors. Biotechnology and conservation of cultural heritage. Springer International Publishing, Switzerland; pp 31-48. 10.1007/978-3-319-46168-7_2
- Górny RL, Harkawy AS, Ławniczek-Wałczyk A, Karbowska-Berent J, Wlazło A, Niesler A, Gołofit-Szymczak M, and Cyprowski M (2016). Exposure to culturable and total microbiota in cultural heritage conservation laboratories. Int J Occup Med Environ Health 29(2): 255-275. 10.13075/ijomeh.1896.00630
- Pinzari F (2011). Microbial ecology of indoor environments. The ecological and applied aspects of microbial contamination in archives, libraries and conservation environments. In: Abdul-Wahab SA, editor. Sick Building Syndrome in Public Buildings and Workplaces. Springer Berlin Heidelberg, Germany; pp 153-178. 10.1007/978-3-642-17919-8_9
- Schweitzer MD, Calzadilla AS, Salamo O, Sharifi A, Kumar N, Holt G, Campos M, and Mirsaeidi M (2018). Lung health in era of climate change and dust storms. Environ Res 163: 36-42. 10.1016/j.envres.2018.02.001
- Maggi O, Persiani AM, Gallo F, Valenti P, and Pasquariello G (2000). Airborne fungal spores in dust present in archives: Proposal for a detection method, new for archival materials. Aerobiologia 16: 429-434. 10.1023/A:1026522826477
- Rintala H, Pitkäranta M, and Täubel M (2012). Microbial communities associated with house dust. Adv Appl Microbiol 78: 75-120. 10.1016/B978-0-12-394805-2.00004-X
- Meklin T, Haugland RA, Reponen T, Varma M, Lummus Z, Bernstein D, Wymer LJ, and Vesper SJ (2004). Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J Environ Monit 6(7): 615-620. 10.1039/b400250d
- Nevalainen A, and Morawska L, editors (2009). Biological agents in indoor environments. Assessment of health risks. Work conducted by a WHO Expert group between 2000-2003. Queensland University of Technology, Australia. Available at: http://www.ilaqh.qut.edu.au/Misc/BIOLOGICAL_AGENTS_2009.pdf [Accessed 06.09.2010].
- Pasquarella C, Saccani E, Sansebastiano GE, Ugolotti M, Pasquariello G, and Albertini R (2012). Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann Agric Environ Med 19(2): 209-212. 22742789
- Awad AHA, Saeed Y, Shakour AA, Abdellatif NM, Ibrahim YH, Elghanam M, and Elwakeel F (2020). Indoor air fungal pollution of a historical museum, Egypt: A case study. Aerobiologia 36: 197-209. 10.1007/s10453-019-09623-w
- Aleksic B, Draghi M, Ritoux S, Bailly S, Lacroix M, Oswald IP, Bailly J-D, Robinec E (2017). Aerosolization of mycotoxins after growth of toxinogenic fungi on wallpaper. Appl Environ Microbiol 83(16): 1-12. 10.1128/AEM.00653-17
- Pinzari F, and Gutarowska B (2021). Extreme colonizers and rapid profiteers: The challenging world of microorganisms that attack paper and parchment. In: Joseph E, editor. Microorganisms in the Deterioration and Preservation of Cultural Heritage. Springer, Switzerland; pp 79-113. 10.1007/978-3-030-69411-1_4
- Karbowska-Berent J, Górny RL, Strzelczyk AB, and Wlazło A (2011). Airborne and dust borne microorganisms in selected Polish libraries and archives. Build Environ 46: 1872-187. 10.1016/j.buildenv.2011.03.007
- Skóra J, and Gutarowska B (2016). Microorganisms in archives and libraries. In: Gutarowska B, editor. A modern approach to biodeterioration assessment and the disinfection of historical book collections. Institute of Fermentation Technology and Microbiology, Lodz University of Technology, Lodz, Polan; pp 4-29.
- Roussel S, Reboux G, Millon L, Parchas MD, Boudih S, Skana F, Delaforge M, and Rakotonirainy MS (2012). Microbiological evaluation of ten French archives and link to occupational symptoms. Indoor Air 22(6): 514-522. 10.1111/j.1600-0668.2012.00781.x
- Osman ME, Abdel-Hameed AA, Ibrahim HY, Yousef F, Abo-Elnasr FF, and Saeed Y (2017). Air microbial contamination and factors affecting its occurrence in certain book libraries in Egypt. Egypt J Bot 57(1): 93-118.
- Pinheiro AC (2014). Fungal communities in archives: Assessment strategies and impact on paper conservation and human health. PhD Thesis, Nova de Lisboa University, Portugal. Available at: https://run.unl.pt/bitstream/10362/14890/1/Pinheiro_2014.pdf. [Accessed 15.12.2016].
- Mallo A, Nitiu D, Elíades L, and Saparrat M (2017). Fungal degradation of cellulosic materials used as support for cultural heritage. Int J Conserv Sci 8(4): 619-632.
- Borrego S, Molina A, and Santana A (2017). Fungi in archive repositories environments and the deterioration of the graphics documents. EC Microbiol 11(5): 205-226.
- Michaelsen A, Piñar G, and Pizari F (2010). Molecular and microscopical investigation of the microflora inhabiting a deteriorated Italian manuscript dated from the Thirteenth century. Microb Ecol 60: 69-80. 10.1007/s00248-010-9667-9
- Simon-Nobbe B, Denk U, Pöll V, Rid R, and Breitenbach M (2008) The spectrum of fungal allergy. Int Arch Allergy Immunol 145(1): 58-86. 10.1159/000107578
- Barnes CS, Alexis NE, Bernstein JA, Cohn JR, Demain JG, Horner E, Levetin E, Nel A, and Phipatanakul W (2013). Climate change and our environment: The effect on respiratory and allergy disease. J Allergy Clin Immunol Pract 1(2): 137-141. 10.1016/j.jaip.2012.07.002
- Guiamet PS, Borrego S, Lavin P, Perdomo I, and Gómez de Saravia S (2011). Biofouling and biodeterioration in material stored at Historical Archive of the Museum of La Plata, Argentine and at the National Archive of the Republic of Cuba. Colloid Surface B 85(2): 229-234. 10.1016/j.colsurfb.2011.02.031
- Lavin P, Gómez de Saravia S, and Guiamet PS (2014). An environmental assessment of biodeterioration in document repositories. Biofouling 30(5): 561-569. 10.1080/08927014.2014.897334
- Borrego S, and Molina A (2020). Behavior of the cultivable airborne mycobiota in air-conditioned environments of three Havanan archives, Cuba. J Atmospheric Sci Res 3(1): 16-28. 10.30564/jasr.v3i1.1910
- Pasquarella C, Balocco C, Saccani E, Capobianco E, Viani I, Veronesi L, Pavani F, Pasquariello G, Rotolo V, Palla F, and Albertini R (2020). Biological and microclimatic monitoring for conservation of cultural heritage: A case study at the De Rossi room of the Palatina library in Parma. Aerobiologia 36(1): 105-111. 10.1007/s10453-019-09610-1
- Pyrri I, Tripyla E, Zalachori A, Chrysopoulou M, Parmakelis A, and Kapsanaki-Gotsi A (2020). Fungal contaminants of indoor air in the National Library of Greece. Aerobiologia 10.1007/s10453-020-09640-0
- Borrego S, and Perdomo I (2012). Aerobiological investigations inside repositories of the National Archive of the Republic of Cuba. Aerobiologia 28(3): 303-316. 10.1007/s10453-011-9235-x
- Borrego S, and Molina A (2014). Comportamiento de la aeromicrobiota en dos depósitos del Archivo Nacional de la República de Cuba durante 7 años de estudio. AUGMDOMUS 6:1-24. Available at: http://revistas.unlp.edu.ar/domus/issue/view/99 [Accessed 10.06.2014].
- Molina A, and Borrego S (2014). Análisis de la micobiota existente en el ambiente interior de la mapoteca del Archivo Nacional de la República de Cuba. Bol Micol 29(1): 2-17.
- Borrego S, and Perdomo I (2016). Airborne microorganisms cultivable on naturally ventilated document repositories of the National Archive of Cuba. Environ Sci Pollut Res 23(4): 3747-3757. 10.1007/s11356-015-5585-1
- Borrego SF, and Molina A (2018). Determination of viable allergenic fungi in the documents repository environment of the National Archive of Cuba. Austin J Public Health Epidemiol 5(3): 1077.
- Borrego S, and Perdomo I (2014). Caracterización de la micobiota aérea en dos depósitos del Archivo Nacional de la República de Cuba. Rev Iberoam Micol 31(3): 182-187. 10.1016/j.riam.2013.09.004
- Borrego S, Guiamet P, Vivar I, and Battistoni P (2018). Fungi involved in biodeterioration of documents in paper and effect on substrate. Acta Microsc 27(1): 37–44.
- Molina A, and Borrego S (2015). El planero como barrera contra agentes biodeteriorantes de mapas y planos. Ph investigación (4): 45-61. Available at: http://www.iaph.es/phinvestigacion/index.php/phinvestigacion/article/view/77 [Accessed 30.06.2015].
- Skóra J, Gutarowska B, Pielech-Przybylska K, Stępień L, Pietrzak K, Piotrowska M, and Pietrowski P (2015). Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia 31(3): 389-401. 10.1007/s10453-015-9372-8
- Rahmawati SL, Zakaria L, and Rahayu ES (2018). The diversity of indoor airborne molds growing in the university libraries in Indonesia. Biodiversitas 19(1): 194-201. 10.13057/biodiv/d190126
- Kadaifciler GD (2017). Bioaerosol assessment in the library of Istanbul University and fungal flora associated with paper deterioration. Aerobiologia 33(1): 151-166. 10.1007/s10453-016-9457-z
- Chen C, and Zhao B (2021) Impact of outdoor particles on indoor air. In: Zhang Y, Hopke PK, and Mandin C, editors. Handbook of Indoor Air Quality. Springer Nature Singapore Pte Ltd, Singapore; pp. 1-23. 10.1007/978-981-10-5155-5_9-1
- Cuesta-Santos O, González-Jaime Y, Sosa-Pérez C, López-Lee R, Bolufé-Torres J, and Reyes-Hernández F (2019). La calidad del aire en La Habana. Actualidad. Rev Cuba Meteorol 25(3): 425-442.
- Chakraborty P, Gupta-Bhattacharya S, Chowdhury I, Majumdar M, and Chanda S (2001). Differences in concentrations of allergenic pollens and spores at different heights on an agricultural farm in West Bengal, India. Ann Agric Environ Med 8(2): 123-130. 11748868
- Li L, Lei C, and Liu Z-G (2010). Investigation of airborne fungi at different altitudes in Shenzhen University. Natural Science 2(5): 506-514. 10.4236/ns.2010.25063
- Stryjakowska-Sekulska M, Piotraszewska-Pająk A, Szyszka A, Nowicki M, and Filipiak M (2007) Microbiological quality of indoor air in university rooms. Pol J Environ Stud 16(4): 623-632.
- Rojas TI, Martínez E, Aira M, and Almaguer M (2008). Aeromicota de ambientes internos: Comparación de métodos de muestreo. Bol Micol 23: 67-73.
- Rodríguez JC, Rodríguez B, and Borrego SF (2014) Evaluación de la calidad micológica ambiental del depósito de fondos documentales del Museo Nacional de la Música de Cuba en época de lluvia. AUGMDOMUS 6: 123-146. Available at: http://revistas.unlp.edu.ar/domus/issue/view/99 [Accessed 10.06.2014].
- Rodríguez JC (2016). Evaluación aeromicrobiológica del depósito del Centro de Documentación del Museo Nacional de la Música de Cuba. Ge-conservación (9): 117-126.
- Rodríguez JC (2016). Microbiología aplicada: Una herramienta para la conservación del Patrimonio Cultural. Conserv Patrim 24: 23-36. 10.14568/cp2015007
- Borrego S, Molina A, and Abrante T (2020). Sampling and characterization of the environmental fungi in the Provincial Historic Archive of Pinar del Río, Cuba. J Biomed Res Environ Sci 1(8): 404-420. 10.30564/jasr.v3i1.1910
- Borrego S, Molina A, and Castro M (2021). Assessment of the airborne fungal communities in repositories of the Cuban Office of the Industrial Property: Their influence in the documentary heritage conservation and the personnel's health. Rev Cub Cien Biol 9(1): 1-18. Available at: http://www.rccb.uh.cu/index.php/RCCB/article/view/312/388 [Accessed 13.09.2021]
- Rojas TI, Aira MJ, Batista A, Cruz IL, and González S (2012). Fungal biodeterioration in historic buildings of Havana (Cuba). Grana 51: 44-51. 10.1080/00173134.2011.643920
- Sánchez KC, Almaguer M, Pérez I, Rojas TI, and Aira MJ (2019). Diversidad fúngica en la atmósfera de La Habana (Cuba) durante tres períodos poco lluviosos. Rev Int Contam Ambie 35(1): 137-150. 10.20937/RICA.2019.35.01.10
- Mallo AC, Nitiu DS, Elíades LA, García M, and Saparrat MCN (2020). Análisis de la carga fúngica en el aire de la sala “Fragmentos de Historia a Orillas del Nilo” y del exterior del Museo de La Plata, Argentina. Ge-conservación (17): 33-46. 10.37558/gec.v17i1.682
- Pinheiro AC, Sequeira SO, and Macedo MF (2019). Fungi in archives, libraries, and museums: a review on paper conservation and human health. Crit Rev Microbiol 45(5-6):686-700. 10.1080/1040841X.2019.1690420.
- Valdés C, Corvo F, González E, Pérez J, Portilla C, and Cuesta O (2007) Mecanismos de deterioro de la piedra caliza coralina estructural del Convento y Basílica Menor de San Francisco de Asís y ensayo de productos para su conservación. Rev CENIC Cienc Quim 38(3): 381-388.
- Niesler A, Górny RL, Wlazło A, Łudzeń-Izbińska B, Ławniczek-Wałczyk A, Gołofit-Szymczak M, Meres Z, Kasznia-Kocot J, Harkawy A, Lis DO, and Anczyk E (2010). Microbial contamination of storerooms at the Auschwitz-Birkenau Museum. Aerobiologia 26: 125-133. 10.1007/s10453-009-9149-z
- OSHA (Occupational Safety and Health Administration) (2002). Indoor air quality investigation. OSHA Technical Manual, Section III, Ch. 2, US Department of Labor, Occupational Safety & Health Administration, Washington DC.
- Alghamdi MA, Shamy M, Redal MA, Khder M, Awad AH, and Elserougy S (2014). Microorganisms associated particulate matter: A preliminary study. Sci Total Environ 479-480: 109-116. 10.1016/j.scitotenv.2014.02.006
- Stamatelopoulou A, Pyrri I, Asimakopoulos DN, and Maggos T (2020). Indoor air quality and dustborne biocontaminants in bedrooms of toddlers in Athens, Greece. Build Environ 173: 106756. 10.1016/j.buildenv.2020.106756
- Almaguer M, Díaz L, Fernández-González M, and Salas S (2021). Assessment of airborne Curvularia propagules in the atmosphere of Havana, Cuba. Aerobiologia 37: 53-69. 10.1007/s10453-020-09674-4
- Harkawy A, Górny RL, Ogierman L, Wlazło A, Ławniczek-Wałczyk A, and Niesler A (2011). Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Ann Agric Environ Med 18(2): 323-329. 22216807
- Domsch KH, Gams W, and Anders TH, editors (1980). Compendium of soil fungi. Vol. 1. Academic Press LTD, London.
- Cai KZ, Liu JL, Liu W, Wang BB, Xu Q, Sun LJ, Chen MY, Zhao MW, and Wu JY (2016). Screening of different sample types associated with sheep and cattle for the presence of nematophagous fungi in China. J Basic Microbiol 56(3): 214-228. 10.1002/jobm.201500281
- Piontelli E, and Díaz MC (2004). El entorno humano y la relevancia biológica de las especies de Neurospora: Consideraciones en Micología Médica. Bol Micol 19:1-11.
- de Hoog GS, Guarro G, Gene J, and Figueras MJ (2000). Atlas of clinical fungi. 2nd edition. Centraalbureau voor Schimmelcultures, Utrecht/Universitat Rovira I Virgili, Reus, Spain.
- Almaguer M and Rojas TI (2013). Aeromicota viable de la atmósfera de La Habana, Cuba. Nova Acta Cient Compostelana (Bioloxía) 20: 35-45.
- Piontelli E (2008). Aportes morfotaxonómicos en el género Aspergillus Link: Claves para las especies ambientales y clínicas más comunes. Bol Micol 23: 49-66.
- Egbuta MA, Mwanza M, and Babalola OO (2016). A review of the ubiquity of ascomycetes filamentous fungi in relation to their economic and medical importance. Advances in Microbiology 6: 1140-1158. 10.4236/aim.2016.614103
- Abdel Hameed AA, Khoder MI, Ibrahim YH, Saeed Y, Osman ME, and Ghanem S (2012). Study on some factors affecting survivability of airborne fungi. Sci Total Environ 414: 696-700. 10.1016/j.scitotenv.2011.10.042
- Almaguer M, Aira MJ, Rodríguez-Rajo FJ, and Rojas TI (2014). Temporal dynamics of airborne fungi in Havana (Cuba) during dry and rainy seasons: Influence of meteorological parameters. Int J Biometeorol 58: 1459-1470. 10.1007/s00484-013-0748-6
- Górny RL (2004). Filamentous microorganisms and their fragments in indoor air. Ann Agric Environ Med 11: 185-197. 15627323
- Micheluz A, Manente S, Tigini V, Prigione V, Pinzari F, Ravagnan G, and Varese G (2015). The extreme environment of a library: Xerophilic fungi inhabiting indoor niches. Int Biodeterior Biodegr 99: 1-7. 10.1016/j.ibiod.2014.12.012
- Sanchis J (2002). Los nueve parámetros más críticos en el muestreo biológico del aire. Rev Tecn Lab 276: 858-862.
- SAS Super 100™ (2001). Microbiological monitoring of the environment. Instruction manual. International Pbi Spa, Milan, Italy.
- Corvo F, Torrens AD, Martín Y, González E, Pérez J, Valdés C, Castañeda A, and Portilla A (2008). Corrosión atmosférica del acero en interiores. Sus particularidades en el clima tropical de Cuba. Rev de Metal 44(2): 129-137.
- Oliva P, García K, Cortez R, Dávila R, Alfaro MR, and Duke V (2001). Monitoreo del Aire. Manual de Laboratorio. Swisscontact, Suiza.
- Ellis MB (1976). More Dematiaceous hyphomycetes. Commonwealth Mycological Institute, England.
- Klich MA, and Pitt JI (1994). A laboratory guide to the common Aspergillus species and their teleomorphs. CSIRO, Division of Food Processing, Australia.
- Barnett HL, and Hunter BB (1998). Illustrated genera of Imperfect fungi. 4th edition. APS Press, Minneapolis.
- Pitt JI (2000). A laboratory guide to common Penicillium species. 3rd edition. CSIRO, Division of Food Processing, Australia.
- Varga J, Frisvad JC, Kocsubé S, Brankovics B, Tóth B, Szigeti G, and Samson RA (2011). New and revisited species in Aspergillus section Nigri. Stud Mycol 69: 1-17. 10.3114/sim.2011.69.01
- Varga J, Frisvad JC, and Samson RA (2011). Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud Mycol 69: 57-80. 10.3114/sim.2011.69.05
- Samson RA, Peterson SW, Frisvad JC, and Varga J (2011). New species in Aspergillus section Terrei. Stud Mycol 69: 39-55. 10.3114/sim.2011.69.04
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin H-D, Dugan FM, Schroers H-J, Braun U, and Crous PW (2010). Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud Mycol 67: 1-94. 10.3114/sim.2010.67.01
- Bensch K, Braun U, Groenewald JZ, and Crous PW (2012). The genus Cladosporium. Stud Mycol 72: 1-401. 10.3114/sim.2010.67.01
- Smith G (1980). Ecology and Field Biology, 2nd edition. Harper & Row, New York.
- Esquivel PP, Mangiaterra M, Giusiano G, and Sosa MA (2003). Microhongos anemófilos en ambientes abiertos de dos ciudades del nordeste rgentine. Bol Micol 18: 21-28.
- Moreno CE (2001). Métodos para medir la biodiversidad. M&T-Manuales y Tesis SEA, Zaragoza.
- Sterflinger K (2010). Fungi: Their role in deterioration of cultural heritage. Fungal Biol Rev 24: 47-55. 10.1016/j.fbr.2010.03.003
- Molina A, Borrego SF, and Ortega BD (2017) Potencialidades biodeteriorantes y patogénicas de hongos anemófilos ambientales frecuentes en ambiente de archivos y museos cubanos. Rev CENIC Cienc Biol 48(3): 069-080.
- Iwatzu T (1984) A new species of Cladosporium from Japan. Mycotaxon 20: 521-533.
–
ACKNOWLEDGMENTS
The authors want to thank the funds given by the Ministry of Science, Technology and Envi-ronment (CITMA) of Cuba (grant number I-2118025001). We would also like to thank Ms. Vivian Figueredo for helping with the English language review.
COPYRIGHT
© 2022

Air- and dustborne fungi in repositories of the National Archive of the Republic of Cuba by Borrego et al. is licensed under a Creative Commons Attribution 4.0 International License.