Back to article: A yeast model for the mechanism of the Epstein-Barr virus immune evasion identifies a new therapeutic target to interfere with the virus stealthiness
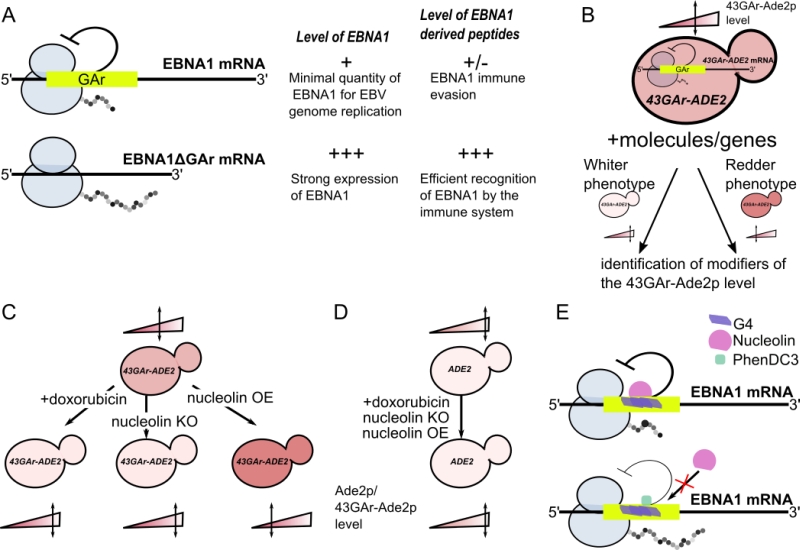
FIGURE 1: Principle of the yeast model for EBNA1 stealthiness and identification of nucleolin as a host factor involved in this process. (A) Schematic representation of the GAr effect in self-limitation of EBNA1 mRNA translation. Thanks to the GAr domain, EBNA1 mRNA is translated at the minimal level to allow EBNA1 protein to fulfill its essential function in EBV genome replication and maintenance and, at the same time, to limit production of EBNA1-derived antigenic peptides. The deletion of GAr leads to a strong production of both EBNA1ΔGAr protein and EBNA1-derived antigenic peptides leading to its detection by the immune system. (B) Rationale of the yeast model for GAr-dependent inhibition of translation. As GAr is also operant in yeast, the 43GAr-Ade2p fusion protein is only weakly expressed in a strain where it is the only source of Ade2p, thereby leading to the formation of pink colonies that express a limited amount of the protein as compared to a control strain expressing a higher Ade2p level, thus forming white colonies. Hence, based on changes in the intensity of its pink color, this strain can be used to look for modifiers that either suppress or exacerbate the GAr inhibitory effect on translation. (C) This 43GAr strain was used for both drug and genetic screening, leading to the isolation of doxorubicin which leads to a GAr-dependent increase in translation and to nucleolin which is a host factor involved in the GAr inhibitory effect on translation. (D) The effect of both doxorubicin and nucleolin are GAr-dependent since they have no effect on the control strain expressing Ade2p. (E) Nucleolin (NCL) role in EBNA1 im-mune evasion involves its ability to bind to G4 formed in the GAr-encoding sequence of the EBNA1 mRNA. PhenDC3, a known G4 ligand, binds efficiently GAr’s G4, thereby preventing NCL binding, which results in loss of GAr inhibitory effect on both translation and antigen presentation.