Back to article: Cristae architecture is determined by an interplay of the MICOS complex and the F1FO ATP synthase via Mic27 and Mic10
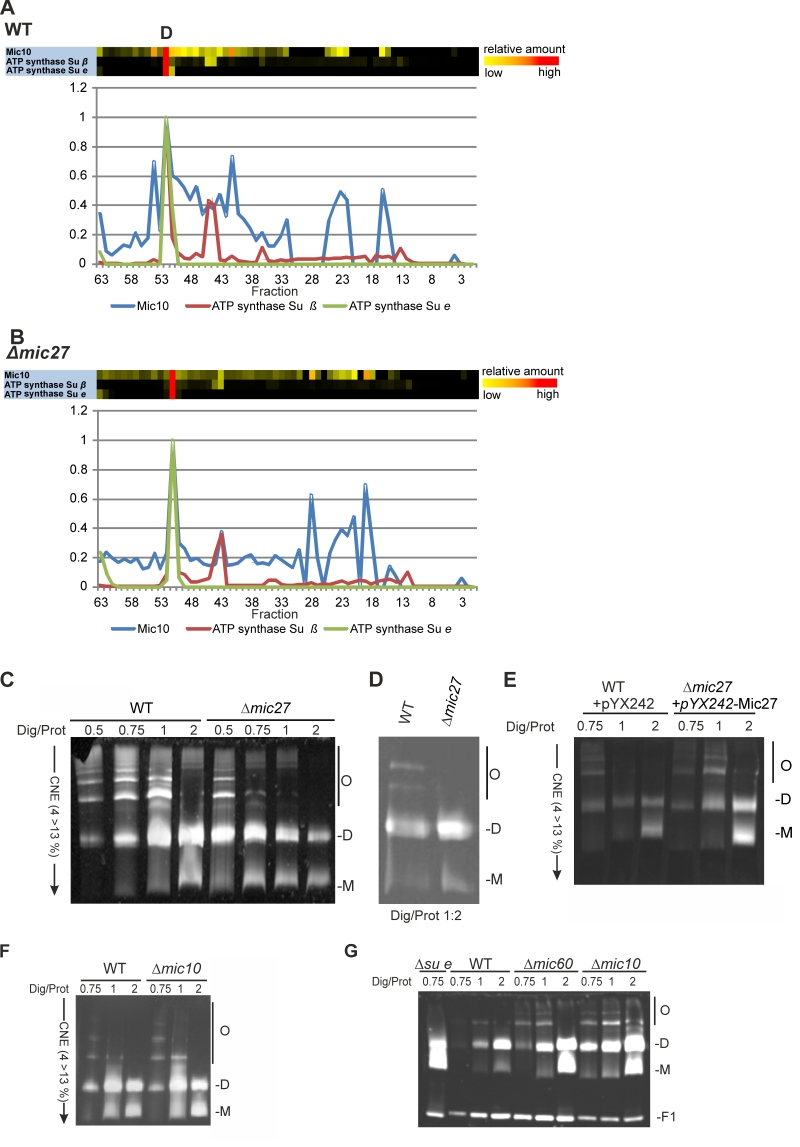
FIGURE 4: Mic27 and Mic10 modulate assembly of F1FO ATP synthase oligomers in an antagonistic manner. (A,B) Mic10 comigrates with the dimeric F1FO ATP synthase irrespective of the presence of Mic27. Complexome distribution profiles of Mic10, F1FO ATP synthase subunit β, and the dimer-specific F1FO ATP synthase subunit e in wildtype (A) and Δmic27 (B) mitochondria (details see Figure 2). (C-E) Mic27 promotes the assembly of the F1FO ATP synthase oligomers. (C,D) Isolated mitochondria of a wildtype (WT) and a Δmic27 yeast strain were solubilized with the indicated ratios of digitonin to protein and separated by CN-PAGE and subsequently in-gel staining of F1FO-ATP synthase activity was performed. (E) Effect of Mic27 overexpression on assembly of the F1FO ATP synthase. Indicated strains were analyzed as in panels C,D. Overexpression of Mic27 was confirmed by Western blot analysis (see SFig. 1C,). (F,G) Mic10 moderately impairs the assembly of the F1FO ATP synthase oligomers. Indicated strains were analyzed as in panels C,D. Strains lacking the F1FO ATP synthase subunit e or Mic60 were used as controls in panel G. Monomers (M), dimers (D), oligomers of the F1FO-ATP synthase (O), and the F1 part (F1) of the F1FO ATP synthase are indicated.