In a nutshell:
Microbial Cell, Vol. 3, No. 3, pp. 101 - 108; doi: 10.15698/mic2016.03.483
Mitochondrial regulation of cell death: a phylogenetically conserved control
1 Equipe 11 labellisée Ligue contre le Cancer, Centre de Recherche des Cordeliers, 75006 Paris, France.
2 INSERM, U1138, 75006 Paris, France.
3 Université Paris Descartes/Paris V, Sorbonne Paris Cité, 75006 Paris, France.
4 Université Pierre et Marie Curie/Paris VI, 75006 Paris.
5 Gustave Roussy Comprehensive Cancer Institute, 94805 Villejuif, France.
6 Metabolomics and Cell Biology Platforms, Gustave Roussy Comprehensive Cancer Institute, 94805 Villejuif, France.
7 Karolinska Institute, Department of Women’s and Children’s Health, Karolinska University Hospital, 17176 Stockholm, Sweden.
8 Pôle de Biologie, Hopitâl Européen George Pompidou, AP-HP; 75015 Paris, France.
Keywords: autophagy, apoptosis, autosis, ferroptosis, MTP-driven regulated necrosis, necroptosis, parthanatos, pyroptosis.
Abbreviations:
MOMP - mitochondrial outer membrane permeabilization,
MPT - mitochondrial permeability transition,
PCD - programmed cell death,
RCD - regulated cell death.
Received originally: 08/01/2016 Accepted: 14/01/2016
Published: 23/02/2016
Correspondence:
Lorenzo Galluzzi, deadoc@vodafone.it
Guido Kroemer, kroemer@orange.fr
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Please cite this article as: Lorenzo Galluzzi, Oliver Kepp and Guido Kroemer (2016). Mitochondrial regulation of cell death: a phylogenetically conserved control. Microbial Cell 3(3): 101-108.
Abstract
Mitochondria are fundamental for eukaryotic cells as they participate in critical catabolic and anabolic pathways. Moreover, mitochondria play a key role in the signal transduction cascades that precipitate many (but not all) regulated variants of cellular demise. In this short review, we discuss the differential implication of mitochondria in the major forms of regulated cell death.
INTRODUCTION
Both prokaryotic and eukaryotic cells succumb to very harsh microenvironmental conditions in a virtually instantaneous and uncontrollable manner. Such form of cellular demise, which has been dubbed “accidental cell death” (ACD), reflects the mechanical disassembly of cellular constituents exposed to excessive temperatures, shear forces and/or pressures, and does not involve any molecular machinery [1]. In addition, both prokaryotes and eukaryotes have evolved systems that precipitate the death of cells experiencing moderate but unresolvable perturbations of intracellular or extracellular homeostasis [2][3]. This latter form of cellular demise, which has been called “regulated cell death” (RCD), relies on the activation of a genetically-encoded machinery, and hence can be modulated by means of pharmacological or genetic interventions [1].
–
Generally, RCD is activated once adaptive response to stress fail at the cellular level, hence constituting a mechanism for the preservation of organismal homeostasis [4][5][6][7]. Defects in the signal transduction cascades that control RCD in eukaryotes have been associated with clinically relevant conditions including acute brain injury, neurodegeneration, cardiac stroke, hepatic damage, and viral infection (all of which are associated with the excessive demise of post-mitotic cells), as well as autoimmune disorders and neoplastic conditions (which are linked to defective RCD) [8][9][10].
–
Of note, one specific variant of RCD that is known as “programmed cell death” (PCD) is initiated at a predetermined point of a cell’s life, as a part of (post-)embryonic development or the maintenance of tissue homeostasis in the adult [1][11]. PCD relies on the same molecular machinery underlying stress-initiated forms of RCD, implying that it can also be retarded or accelerated with specific chemicals or genetic maneuvers [1][11].
–
The signal transduction cascades controlling RCD have expanded considerably throughout evolution, especially (1) once eukaryotic life has been established (i.e., when organelles including mitochondria became available), and (2) along with the transition from a purely unicellular state to multicellularity (through colonial life) [12][13][14]. Nowadays at least five mechanistically distinct variants of RCD have been described in mammals [1][15]: (1) intrinsic apoptosis [16][17][18], (2) extrinsic apoptosis [18][19], (3) necroptosis [20][21][22], (4) mitochondrial permeability transition (MPT)-driven regulated necrosis [22][23][24], and (5) ferroptosis [25][26]. Moreover, other forms or RCD including parthanatos, autosis and pyroptosis are being characterized with increased precision [27][28][29][30][31]. In this short review, we discuss the differential role of mitochondria (which are quintessential for eukaryotic life as they mediate critical bioenergetic and anabolic functions) [32] in the main forms of RCD.
MITOCHONDRIA AND INTRINSIC APOPTOSIS
Intrinsic apoptosis is a form of RCD initiated by perturbations of intracellular homeostasis that relies on the catalytic activity of the cysteine protease caspase-3 (CASP3) [1][15][16][17][18]. In this context, the proteolytic activation of CASP3 is catalyzed by caspase-9 (CASP9), which in turn acquires catalytic activity within a supramolecular complex that is known as “apoptosome” and also contains deoxyATP, the cytosolic adaptor apoptotic peptidase activating factor 1 (APAF1) and an extramitochondrial pool of cytochrome c, somatic (CYCS, best known as CYTC) [33][34].
–
In physiological conditions, CYTC exclusively resides between the outer and the inner mitochondrial membrane, where it is loosely associated with the latter as it operates as an electron shuttle of the respiratory chain [35]. Various perturbations of intracellular homeostasis, however, cause the oligomerization of two members of the Bcl-2 protein family, namely BCL2-associated X protein (BAX)- and BCL2-antagonist/killer 1 (BAK1), in the outer mitochondrial membrane, hence altering its permeability to proteins [36]. Oligomerized BAX and BAK1 also cause rearrangements of the mitochondrial ultrastructure that facilitate the release of CYTC into the cytosol and hence the activation of the apoptosome [33]. Thus, mitochondrial outer membrane permeabilization (MOMP) is a crucial step in the signal transduction cascades that fuel intrinsic apoptosis [36].
–
In line with this notion, several proteins with prominent anti-apoptotic functions, including various other members of the Bcl-2 family like B-cell CLL/lymphoma 2 (BCL2) itself, BCL2-like 1 (BCL2L1, best known as BCL-XL) and myeloid cell leukemia 1 (MCL1), mainly operate by preventing MOMP [37].
–
There are at least two distinct mechanisms whereby BCL2-like proteins mediate such an effect: (1) by physically interacting with BAX and BAK1 and hence preventing their oligomerization [37]; and (2) by sequestering other members of the Bcl-2 protein family that activate BAX and BAK1 in response to stress, the so-called “BH3-only proteins” [38]. Moreover, BCL-XL has been attributed the capacity to retrotranslocate active BAX to the cytosol (where it normally resides in its inactive state) [39].
–
Importantly, MOMP drives intrinsic apoptosis not only as it initiates the apoptosome-dependent activation of CASP3 (which cleaves several substrates that are important for cellular survival), but also because it entails the immediate dissipation of the mitochondrial transmembrane potential (Δψm, which is required for ATP synthesis and several other mitochondrial functions) [40][41]. This implies that intrinsic apoptosis can occur even in the absence of APAF1, CASP9 and CASP3 (or in the presence of chemical agents specifically targeting these proteins) [1]. However, the inhibition of APAF1, CASP9 or CASP3 generally delays intrinsic apoptosis and alters several of its manifestations [1]. Indeed, CASP3 is mechanistically responsible for various biochemical, morphological and immunological features of apoptosis, including the exposure of phosphatidylserine (PS) on the surface of dying cells [42][43], DNA fragmentation (which underlies nuclear condensation) [44][45], and the release of the immunosuppressive factor prostaglandin E2 (PGE2) [46]. In spite of the precise kinetics of the process, mitochondria play a key role in the signal transduction cascades that precipitate intrinsic apoptosis.
MITOCHONDRIA AND EXTRINSIC APOPTOSIS
Extrinsic apoptosis is a CASP3-dependent form of RCD initiated by perturbations of the extracellular microenvironment [1][15][19][47]. Extrinsic apoptosis can be elicited by two classes of plasma membrane receptors that operate in a diametrically opposed fashion: (1) so-called “dependence receptors”, which acquire pro-apoptotic activity when the concentration of their ligands falls below a specific threshold [47]; and (2) so-called “death receptors”, which trigger RCD in the presence of their ligands [19]. The molecular mechanisms bridging dependence receptors to the transmission of an RCD-promoting signal have not been elucidated yet, and appear to exhibit a remarkable degree of context-dependency [47]. Thus, while unbound patched 1 (PTCH1) and deleted in colorectal carcinoma (DCC) appear to interact with the cytosolic adaptor four and a half LIM domains 2 (FHL2, best known as DRAL) to assemble a supramolecular complex that promotes the activation of CASP9 [48][49], other dependence receptors like unc-5 netrin receptor B (UNC5B) have been shown to respond to ligand withdrawal by triggering a death-associated protein kinase 1 (DAPK1)-dependent signaling pathway [50].
–
The signal transduction cascades activated by death receptors upon ligand binding, conversely, are well characterized. Normally, FAS trimers (which assemble and disassemble spontaneously) get stabilized in the presence of FAS ligand (FASLG), favoring the recruitment of a large multiprotein complex at the cytosolic tail of the receptor [19]. This supramolecular entity, which is known as “death-inducing signaling complex” (DISC), contains receptor-interacting protein kinase 1 (RIPK1), FAS-associated protein with a death domain (FADD), various isoforms of CASP8 and FADD like apoptosis regulator (CFLAR, best known as c-FLIP) as well as several members of the baculoviral IAP repeat containing (BIRC) protein family (which act as E3 ubiquitin ligases), and operates as an activating platform for caspase-8 (CASP8) or caspase-10 (CASP10). CASP8 (as well as CASP10) can catalyze the proteolytic activation of CASP3, hence precipitating apoptotic RCD [51][52], while the other components of the DISC either (1) play structural roles (like FADD does), (2) mediate direct RCD-inhibitory functions (like BIRC proteins and c-FLIP do), or (3) connect DISC activation to other signal transduction cascades including the activation of the pro-inflammatory transcription factor NF-κB (like RIPK1 does) [53].
–
Importantly, distinct death receptors assemble structurally different DISCs upon activation, implying that the signaling pathway initiated by death receptors can exhibit a remarkable degree of variation (although they generally culminate in CASP8 or CASP10 activation) [53]. In some cell types (which are commonly referred to as Type I cells, e.g., lymphocytes), the activation of CASP8 by the DISC is perfectly sufficient to drive CASP3-dependent apoptotic RCD [54]. However, in other cell types (which are commonly indicated as Type II cells, e.g., hepatocytes), the optimal activation of CASP3 by CASP8 critically relies on MOMP [54]. In this setting, MOMP is driven by the CASP8-catalyzed activation of BH3 interacting domain death agonist (BID), a potent BH3 only protein [55][56]. Whether cells behave in a Type I or Type II manner upon death receptor ligation depends on the cytosolic abundance of X-linked inhibitor of apoptosis (XIAP), a BIRC family members that exerts potent caspase-inhibitory functions [57]. Thus, mitochondria play an active role in some (but not all) instances of extrinsic apoptosis.
MITOCHONDRIA AND NECROPTOSIS
Necroptosis is a variant of RCD that obligatorily relies on the activation of the RIPK1-like protein receptor-interacting protein kinase 3 (RIPK3) and the pseudokinase mixed lineage kinase domain-like (MLKL), and generally manifests with a necrotic morphology [1][15][20][21][22]. Various (but not all) instances of necroptosis also impinge on the activation of RIPK1 itself, implying that they can be retarded by the RIPK1-targeting agent necrostatin-1 (Nec-1). For instance, this applies to necroptosis elicited by tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) ligation in CASP8-deficient conditions [58][59][60]. Heterotrimeric complexes containing CASP8, FADD and the long isoform of c-FLIP operate indeed as tonic inhibitors of necroptosis, normally preventing the activation of this RCD modality upon death receptor ligation [61][62]. However, when RIPK1 ubiquitination by BIRC family members is chemically antagonized (with agents commonly known as Smac mimetics) and CASP8 is absent or blocked, prolonged TNFRSF1A signaling efficiently drive the assembly of a RIPK1- and RIPK3-containing complex that phosphorylates MLKL, endowing it with the ability to translocate to the inner leaflet of the plasma membrane and compromise its structural integrity [63][64][65][66].
–
Initially, mitochondria were thought to participate in necroptotic signaling in at least two ways: (1) necroptosis was linked to an oxidative burst caused by the RIPK3-dependent activation of various metabolic enzymes, including mitochondrial glutamate dehydrogenase 1 (GLUD1) [67], and (2) MLKL was suggested to boost the catalytic activity of PGAM family member 5, serine/threonine protein phosphatase, mitochondrial (PGAM5), resulting in the activating dephosphorylation of dynamin 1-like (DNM1L, best known as DRP1) and consequent mitochondria fragmentation [68][69]. Subsequent evidence from several independent laboratories, however, demonstrated that mitochondria are completely dispensable for necroptosis. Indeed, necroptotic signaling was found to be normal in cells lacking mitochondria upon a widespread mitophagic response [70], as well as in cells from Pgam5-/- mice [71]. Very recent findings linking MLKL to mitochondrial MCL1 depletion and consequent MOMP remain to be verified [72]. Thus, necroptosis should be considered as a mitochondrion-independent form of RCD.
MITOCHONDRIA AND MPT-DRIVEN REGULATED NECROSIS
The term MPT is commonly employed to indicate an abrupt increase in the permeability of the inner mitochondrial membrane to small solutes, resulting in immediate Δψm dissipation, massive water intake, and osmotic organelle breakdown [1][15][22][23][24]. According to current models, the MPT ensues a conformational change in a multiprotein complex assembled at the juxtaposition between the inner and outer mitochondrial membranes, the so-called “permeability transition pore complex”, (PTPC) [17][24]. The precise molecular composition of the PTPC remains matter of debate and may exhibit considerable degree of context dependency [17][24]. However, at least one protein has been attributed a key, non-redundant role in MPT, i.e., peptidylprolyl isomerase F (PPIF, best known as CYPD) [73][74][75]. Recent findings suggest that also the c subunit of the FO ATPase (which in humans exists in 3 isoforms, ATP5G1-3) plays a critical function within the PTPC [76], yet compelling genetic evidence in support of this hypothesis is difficult to obtain. Irrespective of this unknown, MPT results in a rapid drop of intracellular ATP availability, driving a form of RCD that generally manifests with necrotic morphological features [17][44]. As per definition, MTP-driven regulated necrosis occurs with a delayed kinetics in cells lacking CYPD, as well as in the presence of the chemical CYPD inhibitor cyclosporin A (CsA) [1][15]. Thus, mitochondria play a fundamental role in the signal transduction cascades underlying MPT-driven regulated necrosis.
MITOCHONDRIA AND FERROPTOSIS
Ferroptosis is an iron-dependent form RCD generally initiated by the inhibition of plasma membrane system xC– (a cystine/glutamate antiporter), resulting in the depletion of antioxidant defenses and lethal lipid peroxidation [1][15][25][26]. Ferroptosis is under the endogenous control of cytosolic glutathione peroxidase 4 (GPX4) [77][78], and can be delayed by the small molecule ferrostatin-1 (Fer-1) as well as by other chemical agents that inhibit lipid peroxidation [79].
–
Of note, Fer-1 and alike fail to inhibit the generation of mitochondrial reactive oxygen species (ROS) [79]. Moreover, ferroptosis proceeds normally in Ppif-/- cells as well as in the presence of the MPT inhibitor CsA [80]. Thus, it seems that mitochondria and mitochondrial ROS are perfectly dispensable for ferroptosis, although this conjecture has not yet been addressed experimentally in a direct fashion.
MITOCHONDRIA AND OTHER FORMS OF RCD
Parthanatos
Parthanatos is a peculiar form or RCD depending on poly(ADP-ribose) polymerase 1 (PARP1), a nuclear protein involved in DNA repair, and apoptosis inducing factor, mitochondria associated 1 (AIFM1) [1][15][81]. PARP1 hyperactivation by DNA alkylating agents entails a very pronounced depletion in intracellular NAD+ stores, resulting in a potentially lethal bioenergetic crisis [82]. Moreover, poly(ADP-ribose) moieties generated by PARP1 appear to bind AIFM1 in the mitochondrial intermembrane space, hence favoring its release to the cytosol [83]. Upon binding to peptidylprolyl isomerase A (PPIFA, best known as CYPA), extramitochondrial AIFM1 acquires the ability to translocate to the nucleus and mediate large-scale DNA fragmentation [83]. Mitochondria are therefore required for parthanatos to proceed according to a normal kinetics.
–
Autosis
Autosis is a variant of autophagic cell death, i.e., a form of RCD that is precipitated by the molecular machinery for macroautophagy [1][15][27][28]. In addition, autosis impinges on the plasma membrane Na+/K+ ATPase, implying that it can be modulated with chemical agents that target this ionic pump, like cardiac glycosides [27][84]. The morphological manifestations of autosis differ from those of classical apoptosis and necrosis, encompassing a pathognomonic dilation of the perinuclear space and the massive accumulation of autophagic vacuoles in the cytoplasm [27][28][44]. Although some components of the molecular machinery for macroautophagy interact with mitochondrial proteins (including BCL2), the involvement of mitochondria in the signal transduction cascades that precipitate autosis has not been investigated yet.
–
Pyroptosis
Pyroptosis is a form of RCD that critically rely on the cleavage of gasdermin D (GSDMD) by inflammatory caspases, i.e., caspase-1 (CASP1), caspase-4 (CASP4), caspase-5 (CASP5) or caspase-11 (Casp11, the mouse orthologue of human CASP4 and CASP5) [1][15][29][30][31]. Thus, pyroptosis is generally associated with the assembly and activation of so-called “inflammasomes”, which are supramolecular platforms that promote the CASP1-, CASP4-, CASP5- or Casp11-dependent proteolytic processing of pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) [85]. These observations imply that pyroptosis (1) can only occur in cell types that express sufficient amount of inflammatory caspases (e.g., cells of the monocytic lineage) [86], (2) is associated with the release of mature IL-1β and IL-18 [86], and (3) is sensitive to broad-spectrum caspase inhibitors like Z-VAD-fmk (which also delays apoptosis) as well as to chemicals that specifically block CASP1, CASP4, CASP5 or Casp11 (which have no effects on apoptosis) [1].
–
Morphologically, pyroptosis manifests with features that resemble (at least in part) those of apoptosis [44][87]. Importantly, mitochondrial ROS have been shown to act as intracellular danger signals and promote inflammasome activation coupled CASP1-dependent RCD in some cells [88]. However, the integrity of mitochondria appears to be preserved in the first phases of pyroptotic signaling [89][90][91].
–
In summary, it remains to be formally demonstrated whether mitochondria are a core component of the signal transduction cascades that precipitate pyroptosis or whether they simply act as pyroptosis initiators in specific pathophysiological settings.
CONCLUDING REMARKS
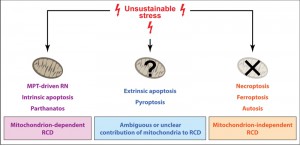
The signal transduction cascades that precipitate RCD have become increasingly more complex with evolution, especially along with the acquisition of the eukaryotic state and multicellularity [12][13][14][92]. Modern prokaryotes harness RCD to favor the survival of the species when colonies are threatened by environmental conditions [93][94], and it seems that such an evolutionarily ancient capacity has been fixed by evolution. Mitochondria (the remnants of bacteria that at some stage were incorporated into protoeukaryotes to generate eukaryotic life) play indeed a fundamental function in some (but not all) RCD-stimulating pathways in modern eukaryotes (Figure 1). Interestingly enough, evolutionarily ancient eukaryotes including Saccharomyces cerevisiae mostly (if not exclusively) rely on mitochondrion-dependent forms of RCD [92][95][96][97]. Conversely, mitochondrion-dependent RCD variants seem to have completely disappeared in post-mitotic animals like Caenorhabditis elegans [98] and Drosophila melanogaster [99]. Taken together, these observations suggest that mitochondrion-dependent variants of RCD may have evolved before their mitochondrion-independent counterparts.
–
In conclusion, mitochondria are quintessential for eukaryotic cells, not only as they mediate fundamental bioenergetic and anabolic functions, but also as they contribute to several (but not all) signal transduction cascades that precipitate RCD.
References
- L. Galluzzi, J.M. Bravo-San Pedro, I. Vitale, S.A. Aaronson, J.M. Abrams, D. Adam, E.S. Alnemri, L. Altucci, D. Andrews, M. Annicchiarico-Petruzzelli, E.H. Baehrecke, N.G. Bazan, M.J. Bertrand, K. Bianchi, M.V. Blagosklonny, K. Blomgren, C. Borner, D.E. Bredesen, C. Brenner, M. Campanella, E. Candi, F. Cecconi, F.K. Chan, N.S. Chandel, E.H. Cheng, J.E. Chipuk, J.A. Cidlowski, A. Ciechanover, T.M. Dawson, V.L. Dawson, V. De Laurenzi, R. De Maria, K. Debatin, N. Di Daniele, V.M. Dixit, B.D. Dynlacht, W.S. El-Deiry, G.M. Fimia, R.A. Flavell, S. Fulda, C. Garrido, M. Gougeon, D.R. Green, H. Gronemeyer, G. Hajnoczky, J.M. Hardwick, M.O. Hengartner, H. Ichijo, B. Joseph, P.J. Jost, T. Kaufmann, O. Kepp, D.J. Klionsky, R.A. Knight, S. Kumar, J.J. Lemasters, B. Levine, A. Linkermann, S.A. Lipton, R.A. Lockshin, C. López-Otín, E. Lugli, F. Madeo, W. Malorni, J. Marine, S.J. Martin, J. Martinou, J.P. Medema, P. Meier, S. Melino, N. Mizushima, U. Moll, C. Muñoz-Pinedo, G. Nuñez, A. Oberst, T. Panaretakis, J.M. Penninger, M.E. Peter, M. Piacentini, P. Pinton, J.H. Prehn, H. Puthalakath, G.A. Rabinovich, K.S. Ravichandran, R. Rizzuto, C.M. Rodrigues, D.C. Rubinsztein, T. Rudel, Y. Shi, H. Simon, B.R. Stockwell, G. Szabadkai, S.W. Tait, H.L. Tang, N. Tavernarakis, Y. Tsujimoto, T. Vanden Berghe, P. Vandenabeele, A. Villunger, E.F. Wagner, H. Walczak, E. White, W.G. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, G. Melino, and G. Kroemer, "Essential versus accessory aspects of cell death: recommendations of the NCCD 2015", Cell Death & Differentiation, vol. 22, pp. 58-73, 2014. http://dx.doi.org/10.1038/cdd.2014.137
- L. Galluzzi, J.M. Bravo-San Pedro, and G. Kroemer, "Organelle-specific initiation of cell death", Nature Cell Biology, vol. 16, pp. 728-736, 2014. http://dx.doi.org/10.1038/ncb3005
- D.R. Green, L. Galluzzi, and G. Kroemer, "Metabolic control of cell death", Science, vol. 345, 2014. http://dx.doi.org/10.1126/science.1250256
- D. Green, and B. Levine, "To Be or Not to Be? How Selective Autophagy and Cell Death Govern Cell Fate", Cell, vol. 157, pp. 65-75, 2014. http://dx.doi.org/10.1016/j.cell.2014.02.049
- C. Hetz, E. Chevet, and H.P. Harding, "Targeting the unfolded protein response in disease", Nature Reviews Drug Discovery, vol. 12, pp. 703-719, 2013. http://dx.doi.org/10.1038/nrd3976
- V. Sica, L. Galluzzi, J. Bravo-San Pedro, V. Izzo, M. Maiuri, and G. Kroemer, "Organelle-Specific Initiation of Autophagy", Molecular Cell, vol. 59, pp. 522-539, 2015. http://dx.doi.org/10.1016/j.molcel.2015.07.021
- L. Galluzzi, F. Pietrocola, J.M. Bravo‐San Pedro, R.K. Amaravadi, E.H. Baehrecke, F. Cecconi, P. Codogno, J. Debnath, D.A. Gewirtz, V. Karantza, A. Kimmelman, S. Kumar, B. Levine, M.C. Maiuri, S.J. Martin, J. Penninger, M. Piacentini, D.C. Rubinsztein, H. Simon, A. Simonsen, A.M. Thorburn, G. Velasco, K.M. Ryan, and G. Kroemer, "Autophagy in malignant transformation and cancer progression", The EMBO Journal, vol. 34, pp. 856-880, 2015. http://dx.doi.org/10.15252/embj.201490784
- L. Galluzzi, K. Blomgren, and G. Kroemer, "Mitochondrial membrane permeabilization in neuronal injury", Nature Reviews Neuroscience, vol. 10, pp. 481-494, 2009. http://dx.doi.org/10.1038/nrn2665
- M.A. Friese, B. Schattling, and L. Fugger, "Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis", Nature Reviews Neurology, vol. 10, pp. 225-238, 2014. http://dx.doi.org/10.1038/nrneurol.2014.37
- C. Brenner, L. Galluzzi, O. Kepp, and G. Kroemer, "Decoding cell death signals in liver inflammation", Journal of Hepatology, vol. 59, pp. 583-594, 2013. http://dx.doi.org/10.1016/j.jhep.2013.03.033
- Y. Fuchs, and H. Steller, "Programmed Cell Death in Animal Development and Disease", Cell, vol. 147, pp. 742-758, 2011. http://dx.doi.org/10.1016/j.cell.2011.10.033
- D.R. Green, and B. Victor, "The pantheon of the fallen: why are there so many forms of cell death?", Trends in Cell Biology, vol. 22, pp. 555-556, 2012. http://dx.doi.org/10.1016/j.tcb.2012.08.008
- C.E. Bender, P. Fitzgerald, S.W.G. Tait, F. Llambi, G.P. McStay, D.O. Tupper, J. Pellettieri, A.S. Alvarado, G.S. Salvesen, and D.R. Green, "Mitochondrial pathway of apoptosis is ancestral in metazoans", Proceedings of the National Academy of Sciences, vol. 109, pp. 4904-4909, 2012. http://dx.doi.org/10.1073/pnas.1120680109
- A. Degterev, and J. Yuan, "Expansion and evolution of cell death programmes", Nature Reviews Molecular Cell Biology, vol. 9, pp. 378-390, 2008. http://dx.doi.org/10.1038/nrm2393
- L. Galluzzi, I. Vitale, J.M. Abrams, E.S. Alnemri, E.H. Baehrecke, M.V. Blagosklonny, T.M. Dawson, V.L. Dawson, W.S. El-Deiry, S. Fulda, E. Gottlieb, D.R. Green, M.O. Hengartner, O. Kepp, R.A. Knight, S. Kumar, S.A. Lipton, X. Lu, F. Madeo, W. Malorni, P. Mehlen, G. Nuñez, M.E. Peter, M. Piacentini, D.C. Rubinsztein, Y. Shi, H. Simon, P. Vandenabeele, E. White, J. Yuan, B. Zhivotovsky, G. Melino, and G. Kroemer, "Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012", Cell Death & Differentiation, vol. 19, pp. 107-120, 2011. http://dx.doi.org/10.1038/cdd.2011.96
- S.W.G. Tait, and D.R. Green, "Mitochondria and cell death: outer membrane permeabilization and beyond", Nature Reviews Molecular Cell Biology, vol. 11, pp. 621-632, 2010. http://dx.doi.org/10.1038/nrm2952
- G. Kroemer, L. Galluzzi, and C. Brenner, "Mitochondrial Membrane Permeabilization in Cell Death", Physiological Reviews, vol. 87, pp. 99-163, 2007. http://dx.doi.org/10.1152/physrev.00013.2006
- R.C. Taylor, S.P. Cullen, and S.J. Martin, "Apoptosis: controlled demolition at the cellular level", Nature Reviews Molecular Cell Biology, vol. 9, pp. 231-241, 2008. http://dx.doi.org/10.1038/nrm2312
- H. Wajant, "The Fas Signaling Pathway: More Than a Paradigm", Science, vol. 296, pp. 1635-1636, 2002. http://dx.doi.org/10.1126/science.1071553
- P. Vandenabeele, L. Galluzzi, T. Vanden Berghe, and G. Kroemer, "Molecular mechanisms of necroptosis: an ordered cellular explosion", Nature Reviews Molecular Cell Biology, vol. 11, pp. 700-714, 2010. http://dx.doi.org/10.1038/nrm2970
- A. Linkermann, and D.R. Green, "Necroptosis", New England Journal of Medicine, vol. 370, pp. 455-465, 2014. http://dx.doi.org/10.1056/NEJMra1310050
- T.V. Berghe, A. Linkermann, S. Jouan-Lanhouet, H. Walczak, and P. Vandenabeele, "Regulated necrosis: the expanding network of non-apoptotic cell death pathways", Nature Reviews Molecular Cell Biology, vol. 15, pp. 135-147, 2014. http://dx.doi.org/10.1038/nrm3737
- L. Galluzzi, O. Kepp, S. Krautwald, G. Kroemer, and A. Linkermann, "Molecular mechanisms of regulated necrosis", Seminars in Cell & Developmental Biology, vol. 35, pp. 24-32, 2014. http://dx.doi.org/10.1016/j.semcdb.2014.02.006
- M. Bonora, M.R. Wieckowski, C. Chinopoulos, O. Kepp, G. Kroemer, L. Galluzzi, and P. Pinton, "Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition", Oncogene, vol. 34, pp. 1475-1486, 2014. http://dx.doi.org/10.1038/onc.2014.96
- A. Linkermann, R. Skouta, N. Himmerkus, S.R. Mulay, C. Dewitz, F. De Zen, A. Prokai, G. Zuchtriegel, F. Krombach, P. Welz, R. Weinlich, T. Vanden Berghe, P. Vandenabeele, M. Pasparakis, M. Bleich, J.M. Weinberg, C.A. Reichel, J.H. Bräsen, U. Kunzendorf, H. Anders, B.R. Stockwell, D.R. Green, and S. Krautwald, "Synchronized renal tubular cell death involves ferroptosis", Proceedings of the National Academy of Sciences, vol. 111, pp. 16836-16841, 2014. http://dx.doi.org/10.1073/pnas.1415518111
- S. Dixon, K. Lemberg, M. Lamprecht, R. Skouta, E. Zaitsev, C. Gleason, D. Patel, A. Bauer, A. Cantley, W. Yang, B. Morrison, and B. Stockwell, "Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death", Cell, vol. 149, pp. 1060-1072, 2012. http://dx.doi.org/10.1016/j.cell.2012.03.042
- Y. Liu, S. Shoji-Kawata, R.M. Sumpter, Y. Wei, V. Ginet, L. Zhang, B. Posner, K.A. Tran, D.R. Green, R.J. Xavier, S.Y. Shaw, P.G.H. Clarke, J. Puyal, and B. Levine, "Autosis is a Na + ,K + -ATPase–regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia", Proceedings of the National Academy of Sciences, vol. 110, pp. 20364-20371, 2013. http://dx.doi.org/10.1073/pnas.1319661110
- Y. Liu, and B. Levine, "Autosis and autophagic cell death: the dark side of autophagy", Cell Death & Differentiation, vol. 22, pp. 367-376, 2014. http://dx.doi.org/10.1038/cdd.2014.143
- N. Kayagaki, I.B. Stowe, B.L. Lee, K. O’Rourke, K. Anderson, S. Warming, T. Cuellar, B. Haley, M. Roose-Girma, Q.T. Phung, P.S. Liu, J.R. Lill, H. Li, J. Wu, S. Kummerfeld, J. Zhang, W.P. Lee, S.J. Snipas, G.S. Salvesen, L.X. Morris, L. Fitzgerald, Y. Zhang, E.M. Bertram, C.C. Goodnow, and V.M. Dixit, "Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling", Nature, vol. 526, pp. 666-671, 2015. http://dx.doi.org/10.1038/nature15541
- J. Shi, Y. Zhao, K. Wang, X. Shi, Y. Wang, H. Huang, Y. Zhuang, T. Cai, F. Wang, and F. Shao, "Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death", Nature, vol. 526, pp. 660-665, 2015. http://dx.doi.org/10.1038/nature15514
- Y. Lim, and S. Kumar, "A single cut to pyroptosis", Oncotarget, vol. 6, pp. 36926-36927, 2015. http://dx.doi.org/10.18632/oncotarget.6142
- L. Galluzzi, O. Kepp, and G. Kroemer, "Mitochondria: master regulators of danger signalling", Nature Reviews Molecular Cell Biology, vol. 13, pp. 780-788, 2012. http://dx.doi.org/10.1038/nrm3479
- X. Liu, C.N. Kim, J. Yang, R. Jemmerson, and X. Wang, "Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c", Cell, vol. 86, pp. 147-157, 1996. http://dx.doi.org/10.1016/s0092-8674(00)80085-9
- H. Zou, W.J. Henzel, X. Liu, A. Lutschg, and X. Wang, "Apaf-1, a Human Protein Homologous to C. elegans CED-4, Participates in Cytochrome c–Dependent Activation of Caspase-3", Cell, vol. 90, pp. 405-413, 1997. http://dx.doi.org/10.1016/s0092-8674(00)80501-2
- L. Galluzzi, O. Kepp, C. Trojel‐Hansen, and G. Kroemer, "Non‐apoptotic functions of apoptosis‐regulatory proteins", EMBO reports, vol. 13, pp. 322-330, 2012. http://dx.doi.org/10.1038/embor.2012.19
- M.C. Wei, W. Zong, E.H. Cheng, T. Lindsten, V. Panoutsakopoulou, A.J. Ross, K.A. Roth, G.R. MacGregor, C.B. Thompson, and S.J. Korsmeyer, "Proapoptotic BAX and BAK: A Requisite Gateway to Mitochondrial Dysfunction and Death", Science, vol. 292, pp. 727-730, 2001. http://dx.doi.org/10.1126/science.1059108
- P.E. Czabotar, G. Lessene, A. Strasser, and J.M. Adams, "Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy", Nature Reviews Molecular Cell Biology, vol. 15, pp. 49-63, 2013. http://dx.doi.org/10.1038/nrm3722
- J.E. Chipuk, and D.R. Green, "How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?", Trends in Cell Biology, vol. 18, pp. 157-164, 2008. http://dx.doi.org/10.1016/j.tcb.2008.01.007
- F. Edlich, S. Banerjee, M. Suzuki, M. Cleland, D. Arnoult, C. Wang, A. Neutzner, N. Tjandra, and R. Youle, "Bcl-xL Retrotranslocates Bax from the Mitochondria into the Cytosol", Cell, vol. 145, pp. 104-116, 2011. http://dx.doi.org/10.1016/j.cell.2011.02.034
- N. Zamzami, P. Marchetti, M. Castedo, D. Decaudin, A. Macho, T. Hirsch, S.A. Susin, P.X. Petit, B. Mignotte, and G. Kroemer, "Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death.", The Journal of experimental medicine, vol. 182, pp. 367-377, 1995. http://dx.doi.org/10.1084/jem.182.2.367
- N. Zamzami, P. Marchetti, M. Castedo, C. Zanin, J.L. Vayssière, P.X. Petit, and G. Kroemer, "Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo.", The Journal of experimental medicine, vol. 181, pp. 1661-1672, 1995. http://dx.doi.org/10.1084/jem.181.5.1661
- K. Segawa, S. Kurata, Y. Yanagihashi, T.R. Brummelkamp, F. Matsuda, and S. Nagata, "Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure", Science, vol. 344, pp. 1164-1168, 2014. http://dx.doi.org/10.1126/science.1252809
- J. Suzuki, D.P. Denning, E. Imanishi, H.R. Horvitz, and S. Nagata, "Xk-Related Protein 8 and CED-8 Promote Phosphatidylserine Exposure in Apoptotic Cells", Science, vol. 341, pp. 403-406, 2013. http://dx.doi.org/10.1126/science.1236758
- G. Kroemer, L. Galluzzi, P. Vandenabeele, J. Abrams, E.S. Alnemri, E.H. Baehrecke, M.V. Blagosklonny, W.S. El-Deiry, P. Golstein, D.R. Green, M. Hengartner, R.A. Knight, S. Kumar, S.A. Lipton, W. Malorni, G. Nuñez, M.E. Peter, J. Tschopp, J. Yuan, M. Piacentini, B. Zhivotovsky, and G. Melino, "Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009", Cell Death & Differentiation, vol. 16, pp. 3-11, 2008. http://dx.doi.org/10.1038/cdd.2008.150
- M. Enari, H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata, "A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD", Nature, vol. 391, pp. 43-50, 1998. http://dx.doi.org/10.1038/34112
- Q. Huang, F. Li, X. Liu, W. Li, W. Shi, F. Liu, B. O'Sullivan, Z. He, Y. Peng, A. Tan, L. Zhou, J. Shen, G. Han, X. Wang, J. Thorburn, A. Thorburn, A. Jimeno, D. Raben, J.S. Bedford, and C. Li, "Caspase 3–mediated stimulation of tumor cell repopulation during cancer radiotherapy", Nature Medicine, vol. 17, pp. 860-866, 2011. http://dx.doi.org/10.1038/nm.2385
- B. Gibert, and P. Mehlen, "Dependence Receptors and Cancer: Addiction to Trophic Ligands", Cancer Research, vol. 75, pp. 5171-5175, 2015. http://dx.doi.org/10.1158/0008-5472.CAN-14-3652
- J. Fombonne, P. Bissey, C. Guix, R. Sadoul, C. Thibert, and P. Mehlen, "Patched dependence receptor triggers apoptosis through ubiquitination of caspase-9", Proceedings of the National Academy of Sciences, vol. 109, pp. 10510-10515, 2012. http://dx.doi.org/10.1073/pnas.1200094109
- F. Mille, C. Thibert, J. Fombonne, N. Rama, C. Guix, H. Hayashi, V. Corset, J.C. Reed, and P. Mehlen, "The Patched dependence receptor triggers apoptosis through a DRAL–caspase-9 complex", Nature Cell Biology, vol. 11, pp. 739-746, 2009. http://dx.doi.org/10.1038/ncb1880
- C. Guenebeaud, D. Goldschneider, M. Castets, C. Guix, G. Chazot, C. Delloye-Bourgeois, A. Eisenberg-Lerner, G. Shohat, M. Zhang, V. Laudet, A. Kimchi, A. Bernet, and P. Mehlen, "The Dependence Receptor UNC5H2/B Triggers Apoptosis via PP2A-Mediated Dephosphorylation of DAP Kinase", Molecular Cell, vol. 40, pp. 863-876, 2010. http://dx.doi.org/10.1016/j.molcel.2010.11.021
- M. Muzio, A.M. Chinnaiyan, F.C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J.D. Bretz, M. Zhang, R. Gentz, M. Mann, P.H. Krammer, M.E. Peter, and V.M. Dixit, "FLICE, A Novel FADD-Homologous ICE/CED-3–like Protease, Is Recruited to the CD95 (Fas/APO-1) Death-Inducing Signaling Complex", Cell, vol. 85, pp. 817-827, 1996. http://dx.doi.org/10.1016/s0092-8674(00)81266-0
- J.P. Medema, "FLICE is activated by association with the CD95 death-inducing signaling complex (DISC)", The EMBO Journal, vol. 16, pp. 2794-2804, 1997. http://dx.doi.org/10.1093/emboj/16.10.2794
- S. Schütze, V. Tchikov, and W. Schneider-Brachert, "Regulation of TNFR1 and CD95 signalling by receptor compartmentalization", Nature Reviews Molecular Cell Biology, vol. 9, pp. 655-662, 2008. http://dx.doi.org/10.1038/nrm2430
- B.C. Barnhart, E.C. Alappat, and M.E. Peter, "The CD95 Type I/Type II model", Seminars in Immunology, vol. 15, pp. 185-193, 2003. http://dx.doi.org/10.1016/s1044-5323(03)00031-9
- H. Li, H. Zhu, C. Xu, and J. Yuan, "Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis", Cell, vol. 94, pp. 491-501, 1998. http://dx.doi.org/10.1016/s0092-8674(00)81590-1
- X. Yin, K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K.A. Roth, and S.J. Korsmeyer, "Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis", Nature, vol. 400, pp. 886-891, 1999. http://dx.doi.org/10.1038/23730
- P.J. Jost, S. Grabow, D. Gray, M.D. McKenzie, U. Nachbur, D.C.S. Huang, P. Bouillet, H.E. Thomas, C. Borner, J. Silke, A. Strasser, and T. Kaufmann, "XIAP discriminates between type I and type II FAS-induced apoptosis", Nature, vol. 460, pp. 1035-1039, 2009. http://dx.doi.org/10.1038/nature08229
- J. Hitomi, D.E. Christofferson, A. Ng, J. Yao, A. Degterev, R.J. Xavier, and J. Yuan, "Identification of a Molecular Signaling Network that Regulates a Cellular Necrotic Cell Death Pathway", Cell, vol. 135, pp. 1311-1323, 2008. http://dx.doi.org/10.1016/j.cell.2008.10.044
- A. Degterev, J. Hitomi, M. Germscheid, I.L. Ch'en, O. Korkina, X. Teng, D. Abbott, G.D. Cuny, C. Yuan, G. Wagner, S.M. Hedrick, S.A. Gerber, A. Lugovskoy, and J. Yuan, "Identification of RIP1 kinase as a specific cellular target of necrostatins", Nature Chemical Biology, vol. 4, pp. 313-321, 2008. http://dx.doi.org/10.1038/nchembio.83
- A. Degterev, Z. Huang, M. Boyce, Y. Li, P. Jagtap, N. Mizushima, G.D. Cuny, T.J. Mitchison, M.A. Moskowitz, and J. Yuan, "Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury", Nature Chemical Biology, vol. 1, pp. 112-119, 2005. http://dx.doi.org/10.1038/nchembio711
- C. Dillon, A. Oberst, R. Weinlich, L. Janke, T. Kang, T. Ben-Moshe, T. Mak, D. Wallach, and D. Green, "Survival Function of the FADD-CASPASE-8-cFLIPL Complex", Cell Reports, vol. 1, pp. 401-407, 2012. http://dx.doi.org/10.1016/j.celrep.2012.03.010
- R. Weinlich, A. Oberst, C. Dillon, L. Janke, S. Milasta, J. Lukens, D. Rodriguez, P. Gurung, C. Savage, T. Kanneganti, and D. Green, "Protective Roles for Caspase-8 and cFLIP in Adult Homeostasis", Cell Reports, vol. 5, pp. 340-348, 2013. http://dx.doi.org/10.1016/j.celrep.2013.08.045
- L. Galluzzi, O. Kepp, and G. Kroemer, "MLKL regulates necrotic plasma membrane permeabilization", Cell Research, vol. 24, pp. 139-140, 2014. http://dx.doi.org/10.1038/cr.2014.8
- X. Chen, W. Li, J. Ren, D. Huang, W. He, Y. Song, C. Yang, W. Li, X. Zheng, P. Chen, and J. Han, "Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death", Cell Research, vol. 24, pp. 105-121, 2013. http://dx.doi.org/10.1038/cr.2013.171
- Z. Cai, S. Jitkaew, J. Zhao, H. Chiang, S. Choksi, J. Liu, Y. Ward, L. Wu, and Z. Liu, "Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis", Nature Cell Biology, vol. 16, pp. 55-65, 2013. http://dx.doi.org/10.1038/ncb2883
- J. Murphy, P. Czabotar, J. Hildebrand, I. Lucet, J. Zhang, S. Alvarez-Diaz, R. Lewis, N. Lalaoui, D. Metcalf, A. Webb, S. Young, L. Varghese, G. Tannahill, E. Hatchell, I. Majewski, T. Okamoto, R. Dobson, D. Hilton, J. Babon, N. Nicola, A. Strasser, J. Silke, and W. Alexander, "The Pseudokinase MLKL Mediates Necroptosis via a Molecular Switch Mechanism", Immunity, vol. 39, pp. 443-453, 2013. http://dx.doi.org/10.1016/j.immuni.2013.06.018
- D. Zhang, J. Shao, J. Lin, N. Zhang, B. Lu, S. Lin, M. Dong, and J. Han, "RIP3, an Energy Metabolism Regulator That Switches TNF-Induced Cell Death from Apoptosis to Necrosis", Science, vol. 325, pp. 332-336, 2009. http://dx.doi.org/10.1126/science.1172308
- Z. Wang, H. Jiang, S. Chen, F. Du, and X. Wang, "The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways", Cell, vol. 148, pp. 228-243, 2012. http://dx.doi.org/10.1016/j.cell.2011.11.030
- L. Sun, H. Wang, Z. Wang, S. He, S. Chen, D. Liao, L. Wang, J. Yan, W. Liu, X. Lei, and X. Wang, "Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase", Cell, vol. 148, pp. 213-227, 2012. http://dx.doi.org/10.1016/j.cell.2011.11.031
- S. Tait, A. Oberst, G. Quarato, S. Milasta, M. Haller, R. Wang, M. Karvela, G. Ichim, N. Yatim, M. Albert, G. Kidd, R. Wakefield, S. Frase, S. Krautwald, A. Linkermann, and D. Green, "Widespread Mitochondrial Depletion via Mitophagy Does Not Compromise Necroptosis", Cell Reports, vol. 5, pp. 878-885, 2013. http://dx.doi.org/10.1016/j.celrep.2013.10.034
- K. Moriwaki, N. Farias Luz, S. Balaji, M.J. De Rosa, C.L. O’Donnell, P.J. Gough, J. Bertin, R.M. Welsh, and F.K. Chan, "The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages", The Journal of Immunology, vol. 196, pp. 407-415, 2016. http://dx.doi.org/10.4049/jimmunol.1501662
- J. Karch, O. Kanisicak, M.J. Brody, M.A. Sargent, D.M. Michael, and J.D. Molkentin, "Necroptosis Interfaces with MOMP and the MPTP in Mediating Cell Death", PLOS ONE, vol. 10, pp. e0130520, 2015. http://dx.doi.org/10.1371/journal.pone.0130520
- C.P. Baines, R.A. Kaiser, N.H. Purcell, N.S. Blair, H. Osinska, M.A. Hambleton, E.W. Brunskill, M.R. Sayen, R.A. Gottlieb, G.W. Dorn, J. Robbins, and J.D. Molkentin, "Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death", Nature, vol. 434, pp. 658-662, 2005. http://dx.doi.org/10.1038/nature03434
- T. Nakagawa, S. Shimizu, T. Watanabe, O. Yamaguchi, K. Otsu, H. Yamagata, H. Inohara, T. Kubo, and Y. Tsujimoto, "Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death", Nature, vol. 434, pp. 652-658, 2005. http://dx.doi.org/10.1038/nature03317
- A.C. Schinzel, O. Takeuchi, Z. Huang, J.K. Fisher, Z. Zhou, J. Rubens, C. Hetz, N.N. Danial, M.A. Moskowitz, and S.J. Korsmeyer, "Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia", Proceedings of the National Academy of Sciences, vol. 102, pp. 12005-12010, 2005. http://dx.doi.org/10.1073/pnas.0505294102
- M. Bonora, A. Bononi, E. De Marchi, C. Giorgi, M. Lebiedzinska, S. Marchi, S. Patergnani, A. Rimessi, J.M. Suski, A. Wojtala, M.R. Wieckowski, G. Kroemer, L. Galluzzi, and P. Pinton, "Role of the c subunit of the FOATP synthase in mitochondrial permeability transition", Cell Cycle, vol. 12, pp. 674-683, 2013. http://dx.doi.org/10.4161/cc.23599
- W. Yang, R. SriRamaratnam, M. Welsch, K. Shimada, R. Skouta, V. Viswanathan, J. Cheah, P. Clemons, A. Shamji, C. Clish, L. Brown, A. Girotti, V. Cornish, S. Schreiber, and B. Stockwell, "Regulation of Ferroptotic Cancer Cell Death by GPX4", Cell, vol. 156, pp. 317-331, 2014. http://dx.doi.org/10.1016/j.cell.2013.12.010
- M. Matsushita, S. Freigang, C. Schneider, M. Conrad, G.W. Bornkamm, and M. Kopf, "T cell lipid peroxidation induces ferroptosis and prevents immunity to infection", Journal of Experimental Medicine, vol. 212, pp. 555-568, 2015. http://dx.doi.org/10.1084/jem.20140857
- R. Skouta, S.J. Dixon, J. Wang, D.E. Dunn, M. Orman, K. Shimada, P.A. Rosenberg, D.C. Lo, J.M. Weinberg, A. Linkermann, and B.R. Stockwell, "Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models", Journal of the American Chemical Society, vol. 136, pp. 4551-4556, 2014. http://dx.doi.org/10.1021/ja411006a
- S.J. Dixon, and B.R. Stockwell, "The role of iron and reactive oxygen species in cell death", Nature Chemical Biology, vol. 10, pp. 9-17, 2013. http://dx.doi.org/10.1038/nchembio.1416
- S.A. Andrabi, T.M. Dawson, and V.L. Dawson, "Mitochondrial and Nuclear Cross Talk in Cell Death", Annals of the New York Academy of Sciences, vol. 1147, pp. 233-241, 2008. http://dx.doi.org/10.1196/annals.1427.014
- F. Cimadamore, C.L. Curchoe, N. Alderson, F. Scott, G. Salvesen, and A.V. Terskikh, "Nicotinamide Rescues Human Embryonic Stem Cell-Derived Neuroectoderm from Parthanatic Cell Death", Stem Cells, vol. 27, pp. 1772-1781, 2009. http://dx.doi.org/10.1002/stem.107
- Y. Wang, N.S. Kim, J. Haince, H.C. Kang, K.K. David, S.A. Andrabi, G.G. Poirier, V.L. Dawson, and T.M. Dawson, "Poly(ADP-Ribose) (PAR) Binding to Apoptosis-Inducing Factor Is Critical for PAR Polymerase-1–Dependent Cell Death (Parthanatos)", Science Signaling, vol. 4, 2011. http://dx.doi.org/10.1126/scisignal.2000902
- L. Menger, E. Vacchelli, S. Adjemian, I. Martins, Y. Ma, S. Shen, T. Yamazaki, A.Q. Sukkurwala, M. Michaud, G. Mignot, F. Schlemmer, E. Sulpice, C. Locher, X. Gidrol, F. Ghiringhelli, N. Modjtahedi, L. Galluzzi, F. André, L. Zitvogel, O. Kepp, and G. Kroemer, "Cardiac Glycosides Exert Anticancer Effects by Inducing Immunogenic Cell Death", Science Translational Medicine, vol. 4, 2012. http://dx.doi.org/10.1126/scitranslmed.3003807
- L. Zitvogel, O. Kepp, L. Galluzzi, and G. Kroemer, "Inflammasomes in carcinogenesis and anticancer immune responses", Nature Immunology, vol. 13, pp. 343-351, 2012. http://dx.doi.org/10.1038/ni.2224
- T. Bergsbaken, S.L. Fink, and B.T. Cookson, "Pyroptosis: host cell death and inflammation", Nature Reviews Microbiology, vol. 7, pp. 99-109, 2009. http://dx.doi.org/10.1038/nrmicro2070
- O. Kepp, L. Galluzzi, L. Zitvogel, and G. Kroemer, "Pyroptosis – a cell death modality of its kind?", European Journal of Immunology, vol. 40, pp. 627-630, 2010. http://dx.doi.org/10.1002/eji.200940160
- P. Gurung, J.R. Lukens, and T. Kanneganti, "Mitochondria: diversity in the regulation of the NLRP3 inflammasome", Trends in Molecular Medicine, vol. 21, pp. 193-201, 2015. http://dx.doi.org/10.1016/j.molmed.2014.11.008
- V. Jesenberger, K.J. Procyk, J. Yuan, S. Reipert, and M. Baccarini, "Salmonella-Induced Caspase-2 Activation in Macrophages", The Journal of Experimental Medicine, vol. 192, pp. 1035-1046, 2000. http://dx.doi.org/10.1084/jem.192.7.1035
- J. Cervantes, T. Nagata, M. Uchijima, K. Shibata, and Y. Koide, "Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages", Cellular Microbiology, vol. 0, pp. 070729204019001-???, 2007. http://dx.doi.org/10.1111/j.1462-5822.2007.01012.x
- S.L. Fink, and B.T. Cookson, "Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages", Cellular Microbiology, vol. 8, pp. 1812-1825, 2006. http://dx.doi.org/10.1111/j.1462-5822.2006.00751.x
- S. Büttner, T. Eisenberg, E. Herker, D. Carmona-Gutierrez, G. Kroemer, and F. Madeo, "Why yeast cells can undergo apoptosis: death in times of peace, love, and war", The Journal of Cell Biology, vol. 175, pp. 521-525, 2006. http://dx.doi.org/10.1083/jcb.200608098
- D. Carmona-Gutierrez, G. Kroemer, and F. Madeo, "When Death Was Young: An Ancestral Apoptotic Network in Bacteria", Molecular Cell, vol. 46, pp. 552-554, 2012. http://dx.doi.org/10.1016/j.molcel.2012.05.032
- D. Dwyer, D. Camacho, M. Kohanski, J. Callura, and J. Collins, "Antibiotic-Induced Bacterial Cell Death Exhibits Physiological and Biochemical Hallmarks of Apoptosis", Molecular Cell, vol. 46, pp. 561-572, 2012. http://dx.doi.org/10.1016/j.molcel.2012.04.027
- T. Eisenberg, S. Büttner, G. Kroemer, and F. Madeo, "The mitochondrial pathway in yeast apoptosis", Apoptosis, vol. 12, pp. 1011-1023, 2007. http://dx.doi.org/10.1007/s10495-007-0758-0
- D. Carmona-Gutierrez, T. Eisenberg, S. Büttner, C. Meisinger, G. Kroemer, and F. Madeo, "Apoptosis in yeast: triggers, pathways, subroutines", Cell Death & Differentiation, vol. 17, pp. 763-773, 2010. http://dx.doi.org/10.1038/cdd.2009.219
- F. Madeo, D. Carmona-Gutierrez, J. Ring, S. Büttner, T. Eisenberg, and G. Kroemer, "Caspase-dependent and caspase-independent cell death pathways in yeast", Biochemical and Biophysical Research Communications, vol. 382, pp. 227-231, 2009. http://dx.doi.org/10.1016/j.bbrc.2009.02.117
- J. Yuan, and H.R. Horvitz, "A first insight into the molecular mechanisms of apoptosis", Cell, vol. 116, pp. S53-S56, 2004. http://dx.doi.org/10.1016/s0092-8674(04)00028-5
- H. Steller, "Regulation of apoptosis in Drosophila", Cell Death & Differentiation, vol. 15, pp. 1132-1138, 2008. http://dx.doi.org/10.1038/cdd.2008.50
COPYRIGHT
© 2016

Mitochondrial regulation of cell death: a phylogenetically conserved control by L. Galluzzi et al. is licensed under a Creative Commons Attribution 4.0 International License.