Reviews:
Microbial Cell, Vol. 3, No. 9, pp. 451 - 475; doi: 10.15698/mic2016.09.529
Recent Insights into the HIV/AIDS Pandemic
1 Department of Medicine, Division of Infectious Diseases, University of California, Irvine, Irvine, CA 92697, USA.
2 Graphic Design, 6850 Dornbirn, Austria.
Keywords: HIV-1, AIDS, antiretroviral therapy, epidemiology, pathology, treatment, virus entry.
Received originally: 17/03/2016 Received in revised form: 25/04/2016
Accepted: 27/04/2016
Published: 05/09/2016
Correspondence:
Johannes S. Gach, PhD, University of California, Irvine, Division of Infectious Diseases Campus, 3501 Hewitt Hall, Irvine, CA 92697-74068, USA; Tel: +1 949-824-3365; Fax: +1 949 824-3365 jgach@uci.edu
Conflict of interest statement: The authors declare that no competing interest exists.
Please cite this article as: Juan C. Becerra, Lukas S. Bildstein, Johannes S. Gach (2016). Recent Insights into the HIV/AIDS Pandemic. Microbial Cell 3(9): 450-474.
Abstract
Etiology, transmission and protection: Transmission of HIV, the causative agent of AIDS, occurs predominantly through bodily fluids. Factors that significantly alter the risk of HIV transmission include male circumcision, condom use, high viral load, and the presence of other sexually transmitted diseases. Pathology/Symptomatology: HIV infects preferentially CD4+ T lymphocytes, and Monocytes. Because of their central role in regulating the immune response, depletion of CD4+ T cells renders the infected individual incapable of adequately responding to microorganisms otherwise inconsequential. Epidemiology, incidence and prevalence: New HIV infections affect predominantly young heterosexual women and homosexual men. While the mortality rates of AIDS related causes have decreased globally in recent years due to the use of highly active antiretroviral therapy (HAART) treatment, a vaccine remains an elusive goal. Treatment and curability: For those afflicted HIV infection remains a serious illness. Nonetheless, the use of advanced therapeutics have transformed a dire scenario into a chronic condition with near average life spans. When to apply those remedies appears to be as important as the remedies themselves. The high rate of HIV replication and the ability to generate variants are central to the viral survival strategy and major barriers to be overcome. Molecular mechanisms of infection: In this review, we assemble new details on the molecular events from the attachment of the virus, to the assembly and release of the viral progeny. Yet, much remains to be learned as understanding of the molecular mechanisms used in viral replication and the measures engaged in the evasion of immune surveillance will be important to develop effective interventions to address the global HIV pandemic.
EPIDEMIOLOGY, INCIDENCE AND PREVALENCE
Acquired immunodeficiency syndrome (AIDS), caused by chronic infection with the human immunodeficiency virus-1 (HIV-1), is one of the most devastating pandemics ever recorded in human history [1]. Shortly after the first reports of AIDS in the United States in 1981 [2][3] and the isolation of HIV-1 two years later [4], the disease has spread relentlessly, infecting close to 80 million people worldwide. The HIV epidemic, which was initially discovered and established in heterosexual populations of Central and East Africa [5][6], arose from zoonotic transmission of simian immunodeficiency virus (SIV) from non-human primates [7], suggesting a much older history of the pandemic [8]. Using statistical approaches on HIV-1 sequence data from Central Africa, it was recently shown that the HIV-1 pandemic ignited in Kinshasa around the early 1920s and that its expansion in Central Africa was contingent upon an active transportation network connecting the country’s main population centers to other regions of sub-Saharan Africa [8].
–
Based on their genetic make-up, HIV-1 viruses are divided into four groups and represent three separate transmission events from chimpanzees (M, N, and O) and one from gorillas (P). Groups N (non-M non-O), O (outlier), and P are restricted to West Africa [9]. Group M (major), which is the main cause of the global HIV pandemic, has diversified into nine subtypes (A-D, F-H, J and K), sub-subtypes (A1-A4 and F1 and F2), and numerous circulating (CRF) and unique recombinant strains (URF). Due to subsequent evolution and spread in the human population there are currently more than 60 CRFs (e.g. CRF01_AE and CRF02_AG) and numerous URF circulating [10]. Subtype C predominates in the actual HIV-1 pandemic with a prevalence of almost 50% followed by subtype A (12%), subtype B (11%), CRF02_AG (8%), CRF01_AE (5%), subtype G (5%) and subtype D (2%). All other subtypes and CRFs represent about 5% of HIV-1 infections in the world [10].
–
The genetic diversity of HIV is primarily caused by the fast replication cycle of the virus coupled with the high error-prone function of its reverse transcriptase [11]. These features allow HIV to evolve around one million times faster than mammalian DNA [12]. Additional genetic diversity is introduced as a result of recombination that takes place during HIV replication when the host cell is infected with multiple HIV-1 subtypes, also known as co-infection or super-infection [13]. Recombination allows for a more rapid increase in viral diversity than does the accumulation of mutations through replication errors. This genetic heterogeneity allows for rapid adaptation to host immune responses, target cell availability, and antiretroviral therapy, which can lead to increased viral pathogenicity, infectivity, and decreased antiretroviral susceptibility [13]. Emerging evidence suggests that clinical progression to AIDS might be more rapid in individuals with dual infection [14].
–
To date more than 40 million people have died due to AIDS-related causes since the pandemic began and millions more are newly infected with the virus each year. In 2014, nearly 37 million people were infected with HIV and the number of people living with HIV continues to increase, in large part because more people globally have access to antiretroviral therapy (ART) [15]. Particularly in the last decade there are signs that the pandemic may be changing course as new HIV infections and AIDS related deaths have significantly declined, contributing to an overall stabilization of the pandemic [16]. For example, as of June 2015, 15.8 million people living with HIV were receiving ART representing over 41% of those in need. Significant progress has also been made in the prevention of mother to child transmission (MTCT) of HIV as 73% of pregnant women living with HIV had access to preventive treatment to protect their babies from infection. Moreover, global incidence has fallen from 3.1 million infections in 2000, to 2 million infections in 2014, representing a decrease of 35% in new infections. Notably, new HIV infections among children have declined by 58% since 2000 [15] and AIDS related deaths have fallen by 42% since the peak in 2004. However, there is still an unacceptably high number of new HIV infections and AIDS-related deaths occurring each year. Alone in 2014, an estimated 2 million people became newly infected with HIV and 1.2 million died of AIDS-related illnesses [17].
–
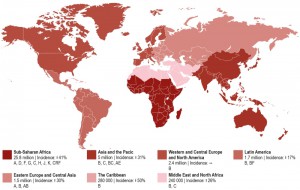
Today, there is no region of the world untouched by this pandemic (Figure 1). Spread of the disease has been particularly alarming in resource-limited countries, especially sub-Saharan Africa and Southeast Asia, but continues to threaten other populations in Eastern Europe, Latin America, and the Caribbean. Nearly 70% of the world’s HIV-infected population lives in sub-Saharan Africa and besides the Caribbean have the highest national rates of adult HIV prevalence (4.7% and 1.1%, respectively) [18]. While the vast majority of new HIV infections in sub-Saharan Africa occur in adults over the age of 25 through heterosexual transmission, HIV disproportionately affects young women [19]. More than 4 in 10 new infections among women are in young women aged 15-24 [20]. The HIV prevalence among females aged 15-19 is eight times higher than that among males at the same age [16]. Sex workers, men who have sex with men (MSM), people who inject drugs (PWID), and children are also key affected populations in sub-Saharan Africa [21]. The highest infection rates were reported for sex workers with an average HIV prevalence of 20%, compared to 3.9% globally and MSM with an HIV prevalence of 15% across Western and Central Africa and 14% across Eastern and Southern Africa [18].
–
Asia and the Pacific represent the second most affected regions by HIV with 14% of the world’s HIV-infected population. However, the region has made tremendous progress in tackling the HIV pandemic reducing the number of new infections by 31% since 2000. Also the adult HIV prevalence rate with 0.2% is relatively low compared to other regions like Western and Central Europe and North America (0.3%), Latin America (0.4%), and Eastern Europe and Central Asia (0.6%) [22]. Risk groups include MSM, PWID, and transgender (TG) populations. The HIV prevalence for the latter population in numerous cities (e.g. Delhi, Phnom Penh, and Mumbai) is much higher than the HIV prevalence in MSM populations. However, MSM remain one of the key affected populations in Asia and the Pacific with rising HIV prevalence [22].
–
North America and Western and Central Europe constitute the third most affected region by the HIV pandemic with 2.4 million people living with HIV. The United States accounts for the majority of people living with HIV in this region (56%). Four countries of Western Europe including France (8%), Spain (6%), United Kingdom (5%), and Italy (5%) contribute an additional quarter of this number [18]. The modes of transmission vary greatly between countries. For example, in 2014, MSM accounted for 44% of new HIV diagnoses in Western Europe and 28% in Central Europe. By comparison, PWID in Central Europe accounted for 5% of new HIV infections compared with 3% in Western Europe. Key affected populations in Western and Central Europe include MSM, migrants from sub-Saharan Africa, PWID and their sexual partners, transgender people, prisoners and sex workers are also at a heightened risk of HIV. In the USA, the majority of newly diagnosed HIV infections in 2013 among adult and adolescent males and females were attributed to MSM and PWID (68%). In contrast, heterosexual contact is thought to contribute 25% of new infections in the United States. African Americans are at a high risk of contracting HIV. In 2013 the infectivity rates were 60% for this racialized group.
–
The Middle East and North Africa has one of the world lowest HIV prevalence rates with 0.1%. However, new HIV infections have risen by 26% since 2000 and AIDS-related deaths increased by 66% since 2005, largely due to the fact that this region has the lowest ART coverage of any region in the world at 11% [18].
–
Recent trends in hospital deaths among HIV-infected patients showed that mortality during ART is often caused by diseases and conditions other than AIDS. According to a recent study, in-hospital deaths among HIV-infected patients declined significantly, and deaths that were not attributable to AIDS increased from 43.0 to 70.5% [23]. Patient factors that were significantly associated with non-AIDS deaths versus AIDS-related deaths included older age (median age, 48 versus 40 years), more likely to be on ART (74.1 versus 55.8%), less likely to have a CD4 count of <200 cells/mm³ (47.2% versus 97.1%), and more likely to have an HIV viral load of ≤400 copies/mL (38.1 versus 4.1%). The most common causes of non-AIDS deaths are non-HIV infection (20.3%), cardiovascular conditions (11.3%), liver disease (8.5%), and malignancies (7.8%) [23]. Notably, the risk of myocardial infarctions in HIV infected people is 50% higher than in people without HIV [24]. In addition, co-infection with hepatitis B (HBV) and C (HCV), which share similar routes of transmission with HIV, is more likely in HIV infected people [25]. For example co-infection with HIV and HCV is very common (50%–90%) among HIV-infected injection drug users in the US [26].
–
HIV associated tuberculosis (TB) remains a global public health challenge among people living with HIV, accounting for around one in three AIDS-related deaths worldwide. A retrospective cohort study conducted in South Africa revealed that TB doubled within the first year after HIV infection [27], thereafter the incidence increased as immunity decreased, and reached a very high prevalence of 25.7 per 100 person-years in patients with CD4 T-cell counts lower than 50 cells per μL [28]. However, TB-related deaths in people living with HIV have fallen by 32% since 2004 [29].
ETIOLOGY, TRANSMISSION AND PROTECTION
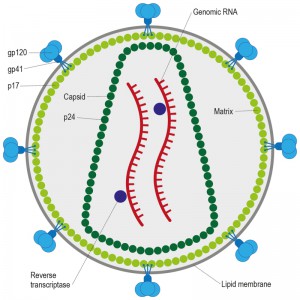
HIV, the causative agent of AIDS, belongs to a class of viruses known as retroviruses and a subgroup of retroviruses known as lentiviruses or “slow” viruses [30]. HIV is an enveloped, single-stranded positive-sense RNA virus (Figure 2) with a genome of 9749 nucleotides in length that encodes a total of nine viral proteins [31]. The HIV genome contains three major genes including gag, pol, and env, encoding major structural proteins as well as essential enzymes (Figure 3). The gag gene encodes viral core proteins, the pol gene encodes a set of enzymes required for viral replication, and the env gene encodes the viral surface glycoprotein gp160 [32]. In addition to these three major proteins, HIV also encodes proteins with certain regulatory and auxiliary functions containing Tat and Rev, which activate viral transcription and control the splicing and nuclear exports of viral transcripts, respectively [33]. Four other genes encode accessory proteins Vif, Vpr, Vpu and Nef, which are not essential for replication in certain tissues. The viral genome is flanked by LTRs (long terminal repeats) that are required for viral transcription, reverse transcription and integration (Figure 3) [34]. The genome dimerization and packaging signal ‘Ψ’ is located between the 5′-LTR and the gag gene [35].
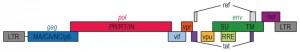 | Figure 3: HIV-1 genome. A schematic representation of the HIV-1 gene products encoded by the HIV-1 genomic sequence. |
–
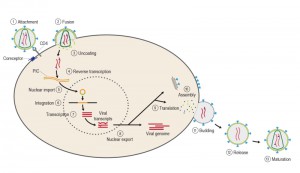
The course of infection with these viruses is typically characterized by a long period between initial infection and the onset of serious symptoms. Like all viruses, HIV can reproduce only inside cells by hijacking the cell’s machinery (Figure 4). Once inside the cell, HIV and other retroviruses use the enzyme reverse transcriptase (RT) to convert their viral RNA into DNA, which can be incorporated into the host cell genome [36]. Once integrated, the proviral DNA is replicated along with cellular DNA during cycles of cell division, as with any cellular gene. The provirus serves as the template for transcription of viral RNAs. Some viral RNAs are translated to yield the viral proteins, whereas a portion of the full-length viral RNA is recruited to serve as genomic RNA in progeny virions [37].
–
Transmission of HIV requires contact with a body fluid that contains either infectious virus (virions) or HIV-infected cells or a combination of both [38]. HIV can appear in nearly any body fluid [39], but transmission occurs predominantly through blood, semen, vaginal and rectal fluids, and breast milk [40]. Although tears, urine, and saliva may contain low concentrations of HIV, transmission through these fluids is extremely rare, if it occurs at all. No case of HIV transmission has been traced to the coughing or sneezing of an infected person or to a mosquito bite. The three main routes of HIV transmission are parenteral exposure (e.g. blood transfusion, needle sharing), unprotected sexual contact, and vertical (mother to child) trans-mission [41]. Sexual exposure is the most common route of infection and drives the HIV pandemic in most countries, followed by needle sharing injective drug use, and MTCT [42]. Based on a recent study [43] analyzing the per-act HIV transmission risk estimates (Table 1) the authors found that blood transfusion ranked on top, followed by vertical transmission, receptive anal intercourse, needle-sharing injection drug use, percutaneous needle stick injuries, insertive anal intercourse, receptive penile–vaginal intercourse, and insertive penile–vaginal intercourse. Although biologically plausible, the transmission risk for receptive and insertive oral sex is relatively low as the oropharynx is considerably less susceptible to HIV infection than the cervico-vaginal environment or penis. This might be due to the thicker epithelial layer of the oropharynx, the low number of CD4+ lymphocytes, and the presence of HIV-specific antibodies and various endogenous factors that inhibit HIV transmission [40][43].
–
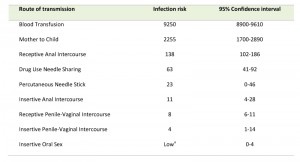 | Table 1. Estimated per-act HIV transmission risk per 10,000 exposures. Adapted from [43]. a Risk is considered to be low relative to the other sexual exposures, but it is not zero. |
Anal intercourse carries a higher risk of HIV transmission for both receptive and insertive partners when compared with vaginal intercourse [44]. The risk of HIV transmission to the receptive partner resulting from receptive anal intercourse is almost 18 times higher than the risk from receptive vaginal intercourse [45]. The higher risk of infection is thought to result from the differences between the tissues involved. First, rectal mucosa is characterized by a higher density of lymphoid follicles, which are overlaid with microfold cells (M cells) that are specialized in antigen uptake. Second, M cells form intraepithelial pockets containing CD4+ memory T cells, macrophages, and dendritic cells (DCs) in close proximity, which could greatly facilitate HIV replication. Third, the single layer of epithelial cells in the rectum could be more susceptible to abrasions than the vaginal mucosa [46][47].
–
One of the most important factors that increase the risk of sexual transmission of HIV-1 is the viral load (i.e. number of viral RNA copies per mL of plasma) [48][49]. During primary infection, the number of HIV-1 particles in plasma increases rapidly, reaches a peak (median 5.8 log10 HIV-1 copies/mL), and then declines until it reaches a set point level [50]. It has been reported that the per act risk of heterosexual transmission of HIV in serodiscordant couples is 2.9 fold (95% CI, 2.2-3.8) increased for each 1.0 log10 increase of the viral load. In contrast, a reduction in plasma viral load of 0.7 log10 is estimated to reduce HIV-1 transmission by 50% [51]. Moreover, higher genital HIV-1 RNA concentrations are also associated with greater risk of heterosexual HIV-1 transmission, and this effect is independent of plasma HIV-1 concentrations, which would make HIV-1 RNA in genital secretions a useful marker of HIV-1 sexual transmission risk [49]. The stage of infection is also an important variable for infectivity, largely because of the accelerated rate of viral shedding during the acute stage [52]. Viral loads in all fluids and tissues, including blood and genital secretions, peak around 4 weeks after viral exposure. The risk of sexual transmission of HIV during this stage is 30–300 times the risk during the post-acute phase of infection, when antibodies and cytotoxic T cell lymphocytes directed against HIV appear [52]. Based on a study conducted in Uganda with HIV-discordant couples [53] it was found that the rate of heterosexual HIV transmission per coital act was highest during early-stage infection, a time when only few seroconverters know their HIV status or receive ART. It is believed that patients with early-stage HIV infection make a highly disproportionate contribution to the incidence of HIV infection [52].
–
Sexually transmitted diseases (STDs) (e.g. genital ulcer disease of any cause, herpes simplex type-2 (HSV-2) infection, and bacterial vaginosis) can also greatly increase both infectivity and susceptibility of HIV [54]. On the susceptibility side, STDs can reduce the efficacy of physical and mechanical barriers of the virus (e.g., by causing lesions or aberrations in the mucosa) [55], increase the number of HIV receptor cells or the number of receptors per cell (e.g. by causing persistent inflammation) [56], and produce a vaginal environment that is more conducive to transmission (e.g. via presence of bacterial vaginosis and increased levels of anaerobic bacteria or various amines) [57][58]. On the infectivity side, STDs might evoke more infectious HIV variants and thus increased HIV concentrations in genital lesions, semen, or both [59]. Moreover, co-transmission of HIV and another STD appears to be a common occurrence [60]. Specific host factors may also augment the risk of HIV transmission [61]. For example, the risk of HIV transmission is elevated 2-3 times in women with cervical ectopy, a condition that renders cervical tissues more vulnerable [62], while in men, the presence of foreskin has been associated with increased risk of HIV acquisition [63]. In addition any factor that increases the opportunity for HIV to reach receptive immune cells may amplify the risk of HIV transmission. For example, hormonal contraceptives have been implicated in several studies, because of their association with vaginal thinning or cervical ectopy [64][65][66]. Frequent use of spermicides containing N-9 has been associated with the disruption and irritation of the genital epithelium that may increase the risk of HIV infection [67][68]. The presence of blood during sexual intercourse, including blood associated with menstruation, has been linked with an increased risk of HIV transmission, particularly from females to males [52].
–
Prevention of sexual HIV transmission has been a priority since the beginning of the epidemic. To control the epidemic many interventions are necessary as no isolated prevention intervention is effective enough on its own. Prevention interventions that can significantly decrease risk of viral infection include: 1) use of antiretroviral drugs for treatment, 2) pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP), 3) male condom use, and 4) medical male circumcision [69][70][71]. The most potent intervention to reduce sexual transmission of HIV is ART. Findings of the landmark HIV Prevention Trials Network (HPTN) 052 trial revealed that immediate ART treatment in serodiscordant couples reduced HIV transmission in the uninfected partner by 96% due to universal viral suppression [72]. However, results of HPTN 052 also clearly displayed the demand for integral prevention interventions, as 25% of HIV transmissions were not from the HIV infected partner [72]. A population-based prospective cohort study in rural KwaZulu-Natal, South Africa, with a total of 16,667 HIV negative participants demonstrated that the risk of HIV infection was associated with ART coverage in the local community [73]. For example, an HIV-uninfected individual living in a community with high ART coverage (30 to 40% of all HIV-infected individuals on ART) was 38% less likely to acquire HIV than someone living in a community where ART coverage was low (<10% of all HIV-infected individuals on ART) [73]. The population-level effects resulting from early ART initiation independent of CD4 counts, the so-called test and treat strategy, are currently being studied in several randomized trials [74]. One of the studies reported a 90% reduction of HIV transmission due to early ART initiation among seronegative sex partners in stable or casual relationships with seropositive individuals. [75]. The feasibility of achieving benefits of ART will need effective interventions to greatly increase the awareness of HIV status. HIV testing and counseling is the first crucial step for linkage to HIV treatment and prevention. However, conventional facility-based HIV-testing and counseling (HTC), although important, has not achieved high testing coverage in sub-Saharan Africa due to limitations in the health care system [76][77]. A meta-analysis [78] found that community HTC had high coverage and uptake and identified HIV-positive people at higher CD4 counts than facility testing. Mobile HIV testing reached the highest proportion of men of all modalities examined (50%, 95% confidence interval (CI) = 47–54%), while self-testing at home reached the highest proportion of young adults (66%, 95% CI = 65–67%). Key populations (commercial sex workers and MSM) yielded high HIV positivity (38%, 95% CI = 19–62%) combined with the highest proportion of first-time testers (78%, 95% CI = 63–88%), indicating service gaps [78]. Community testing with facilitated linkage (for example, counselor follow-up to support inclusion into the program) achieved high access to care (95%, 95% CI = 87–98%) and antiretroviral initiation (75%, 95% CI = 68–82%). Expanding home and mobile testing, self-testing and outreach to key populations with facilitated linkage can increase the proportion of men, young adults and high-risk individuals linked to HIV treatment and prevention, and decrease HIV burden [78]. However, a cautionary point about the introduction of the test and treat strategy is that it could increase antiretroviral resistance [79]. Moreover, optimism about the treatment or misperceptions about the effects of antiretroviral drugs may also cause some people to increase their risk behavior [80]. Meta-analyses of studies conducted in several different risk groups (e.g. MSM, PWID, sex workers) revealed that behavioral interventions reduced self-reported risk behavior. For example, findings of a study of African heterosexual serodiscordant couples showed that self- reported condom use shortened the per-coital act risk of HIV transmission by 78%. [51] A combination of antiretroviral treatment and condom use could theoretically reduce the per-act HIV transmission risk of anal and vaginal intercourse by up to 99.2% [43]. Disclosure of HIV status to sexual partner is an important prevention goal that increases the awareness of HIV risk to untested partners thus leading to greater acceptance of HIV counseling and testing as well as positive changes in risk behaviors [81]. The effect of PrEP on HIV acquisition has been studied in several clinical trials [82][83][84][85]. A once-daily oral dose of tenofovir (TFV) or TFV plus emtricitabine effectively reduced HIV acquisition by 44% to 75%. However, efficacy was strongly associated with adherence to the intervention program [86]. A very recent “on-demand PrEP” study conducted in men who have unprotected anal sex with men revealed that a combination of TFV and emtricitabine, taken before and after sexual activity, provided notable protection against HIV acquisition with a relative reduction of 86% in the risk of HIV-1 infection [87].
–
Medical male circumcision (MC) on the other hand is not only cost saving but also very effective in reducing HIV acquisition in men. A randomized trial with circumcised men in Uganda revealed that HIV incidence over 24 month was 0.66 cases per 100 person-years in the intervention group (MC) versus 1.33 cases per 100 person-years in the control group who were not circumcised [88]. A similar result was found in Kenya, where the risk of HIV acquisition of circumcised men was reduced by 53% [89]. Furthermore, the rate of male-to-female HIV transmission after MC is reduced by 46% [90]. MC also decreases HSV-2 and human papillomavirus (HPV) infection among heterosexual men and provides benefits to female partners, including reduced prevalence of genital ulcer disease, bacterial vaginosis, and HPV [91]. Despite the potential public health benefits of medical MC, there are several scale-up challenges. Many strategies are needed to increase demand for medical MC, including promotion of benefits of circumcision to men and their female partners, and supply-side interventions to provide medical MC through mobile clinics and devices that reduce procedure time [91].
–
Topical PrEP application of vaginal and rectal microbicides is an attractive intervention because, unlike condoms, they are under the control of the receptive partner. Vaginal application of 1% TFV gel demonstrated complete protection from SHIV infection in macaques, when applied 30 min before viral challenge [92]. However, to date there is no real evidence of protection against HIV with the exception of the findings of the CAPRISA 004 trial in South Africa where pericoital use of 1% TFV gel reduced HIV acquisition by 39% [93]. Unfortunately, the result of the CAPRISA 004 study could not be reproduced in a more recent confirmatory trial called FACTS 001 [94][95]. Among the 2029 women in the study allocated to either TFV or placebo gel, there were 61 infections in women using TFV gel and 62 in women using placebo [95]. The use of 1% TFV as a rectal microbicide -applied daily or before and/or after sex to prevent HIV- in MSM and transgender women was recently assessed in a phase 2 extended safety and acceptability study (MTN-017). Based on the results presented at CROI 2016 there was no difference in adherence between sex-based rectal gel use and a daily oral PrEP option [96]. It remains to be seen in upcoming phase III clinical trials whether topical application of rectal microbicides result in a similar efficacy as orally administered PrEP or not. Newer strategies in microbicide development are focused on the sustained delivery of antiretroviral drugs. The dapivirine vaginal ring for HIV-1 prevention in women, which delivers low doses of the NNRTI dapivirine over a month of use, is one example [97]. The efficacy of the dapivirine vaginal ring has been recently tested in a phase III clinical trial conducted in Malawi, South Africa, Uganda, and Zimbabwe. A total of 168 HIV-1 infections occurred among the 2629 women who were enrolled in the trial. The dapivirine group had a 27% lower incidence rate (3.3 cases per 100 person-years; 71 infections) than the placebo group (4.5 cases per 100 person-years; 97 infections) [98].
–
Other interventions to reduce HIV infectivity have focused on treatment of co-infections, notably HSV-2 infection, which causes genital herpes [99]. Although aciclovir and valaciclovir treatment effectively reduce plasma and genital HIV concentrations no association with decreased HIV transmission has been found, except slightly delayed HIV disease progression [100]. Additional research is needed to determine the aspect of alternative interventions to treat co-infections.
–
Substantial progress has been made in the risk reduction of perinatal HIV-1 transmission. MTCT of HIV is relatively rare during early pregnancy and relatively frequent in late pregnancy and during delivery. Knowledge about the timing of HIV-1 transmission to infants has allowed the development of appropriate interventions [99]. Without any intervention, the estimated risk of perinatal transmission ranges from 15% to 40% [101], depending on maternal risk factors (e.g. plasma and breast milk viral load, maternal immunologic status and clinical stage) and whether breastfeeding is practiced [102]. In sub-Saharan Africa, where prolonged breastfeeding is customary, breast milk transmission represents an important mechanism of MTCT of HIV-1 [103]. In a data meta-analysis of more than 3,000 breastfeeding infants of HIV-1-infected women from sub-Saharan Africa, rates of HIV infections through breast milk were estimated with relatively high precision. The study found that the cumulative probability of late postnatal transmission at 18 months was 9.3% and the overall risk of late postnatal transmission was 8.9 transmissions per 100 child-years of breastfeeding [104]. Whether breast-feeding is “exclusive” or “mixed” has also been shown to be of particular importance in the risk of MTCT. Exclusive breast-feeding has been found to have a significantly lower transmission risk than mixed feeding [101]. This is thought to be due to a disruption in the integrity of the intestinal mucosa, which is normally protected by breast milk, allowing HIV to more readily penetrate these microabrasions [105].
–
Analyses of viral load levels from several studies demonstrated a direct positive correlation between maternal RNA viral load in plasma and risk of transmission to the infant [106]. High levels of virus in plasma, and presumably also in breast milk, are observed in primary HIV infection, when the rate of postnatal transmission has been estimated to be nearly 30% [107]. This correlation is also observed among women receiving ART. For example, in a study conducted in the United Kingdom and Ireland with over 10,000 HIV-positive pregnant women, MTCT rates were lower among women who had a viral load <50 copies/mL near delivery, compared with women who had higher viral loads (0.09 percent transmission versus 1.0 and 2.6 percent with viral load ranges 50-399 copies/mL and 400-999 copies/mL, respectively) [108].
–
Additional maternal and infant factors that have been associated with higher risk of transmission are 1) low CD4 cell counts, 2) anemia, 3) more advanced WHO clinical disease stage, 4) maternal mastitis, and 5) acute maternal seroconversion during pregnancy or breastfeeding [109][110]. As an example, in a recent meta-analysis, which included data from 19 cohorts and 22,803 total person-years, the pooled HIV incidence rate during pregnancy/postpartum was 3.8 events per 100 person years, and the pooled risk of MTCT among such women was 23 percent [111]. Recommended MTCT interventions, predominantly ART, have resulted in a ten-fold reduction in this risk, and complete elimination of MTCT is now feasible [99]. Antiretroviral combination therapy is more effective at prevention of MTCT than zidovudine plus one dose of nevirapine, and has the additional advantages of reducing sexual HIV transmission and reducing HIV-associated morbidity and mortality [99]. ART treatment should ideally be started after the first trimester, provided that women are well enough to delay their treatment [112]. However, ART alone will not reach the goal of elimination of prevention of MTCT. Access to prenatal care, HIV testing, and MTCT interventions will need to be substantially increased in regions with high HIV prevalence [112].
–
Despite a number of successful prevention interventions that have been reported, including PrEP prophylaxis and treatment as prevention, ultimate control of the HIV epidemic will most likely come only with the development of a safe and effective preventive vaccine. However, this goal has proved to be elusive [113]. Of the reported HIV vaccine efficacy trials [114][115][116][117][118] only the RV144 HIV vaccine trial consisting of a recombinant Canarypox virus based vector (ALVAC-HIV) and a recombinant envelope glycoprotein gp120-subunit vaccine (AIDSVAX B/E) has been successful in reducing HIV incidence by 31% [119][120][121]. In contrast, the HVTN 505 vaccine study consisting of a DNA prime–recombinant adenovirus type 5 boost (DNA/rAd5) regimen conducted in the US revealed a vaccine efficacy of -25% after a first evaluation with 27 infections in the vaccine group and 21 infections in the placebo group [113]. The data and safety monitoring board stopped further vaccinations due to the fact that the DNA/rAd5 vaccine regimen did not reduce either the rate of HIV-1 acquisition or the viral-load set point in the population studied [113]. Nevertheless, the partial success of the RV144 study has refueled the vaccine field and has led to the development of a vaccine protocol (HIV Vaccine Trials Network (HVTN) 100) to assess the efficacy of this strategy against clade C HIV in South Africa sponsored through the National Institute of Allergy and Infectious Diseases.
PATHOLOGY/SYMPTOMATOLOGY
HIV-1 infection is usually initiated with a single virion infecting a single target cell at the site of entry. Mucosal surfaces represent the number one entry site of HIV as the vast majority of HIV infections result from mucosal transmissions [122]. Mucosal transmission is dependent upon transfer of infectious virus and/or cells across the mucosal epithelium providing access to sub-epithelial DCs, macrophages, monocytes, and/or CD4 T-cells [123][124]. CD4-independent HIV infection of cells has been reported in several occasions involving astrocytes and renal epithelial cells where HIV gene expression plays an important role in the pathogenesis of HIV-associated neurocognitive disorder related to astrocytes and nephropathy related to epithelial cells [125] [126].
–
Numerous mechanisms for mucosal HIV-1 transmission have been proposed including: (i) direct HIV-1 infection of epithelial cells, (ii) transcytosis of HIV-1 across epithelial barriers and/or specialized M cells, (iii) epithelial transmigration of HIV-1-infected donor cells, (iv) uptake of HIV-1 by intra-epithelial Langerhans and dendritic cells, (v) or entry via epithelial micro-abrasions or ulceration [127][128]. Non-human primate studies have revealed that mucosal infection can occur within a very short time period. Following 30-60 min exposure to infectious virus, local infection is established within 16-72 hours, and expansion to draining lymph nodes is accomplished within 24-72 hours [129][130].
–
Establishment of HIV infection across mucosal membranes most often results from the transmission and subsequent propagation of a single virus strain, termed transmitted/founder (T/F) virus [131]. This was demonstrated by the isolation of a single viral genome and its unique viral envelope (Env) in 102 acute HIV-1 infected subjects, where 78 subjects had evidence of productive clinical infection by a single virus, whereas the remaining 24 subjects were infected by a minimum of two to five viruses [132].
–
Several distinctive phenotypic characteristics of T/F virions are clearly associated with transmission: Chemokine receptor 5 (CCR5) tropism, CD4+ T cell tropism, enhanced cell-free infectivity, higher Env content, improved DC interaction, and relative IFN-α resistance [133][134][135][136]. Other phenotypic and genotypic attributes that have been linked to transmission are: Enhanced CD4 receptor and/or coreceptor engagement, increased sensitivity to neutralizing antibodies, Env interaction with integrin pair-α4β7, fewer putative N-linked glycosylation sites, and shorter variable loops [137][138][139][140][141][142][143]. However, to date, no consistent phenotypic correlate of these genetic signatures has been identified [133][144].
–
Although a typical time course of infection can vary considerably from individual to individual, as can the levels of viremia, the general outline is essentially the same in virtually every infected person who does not receive effective ART therapy (Figure 5A) [145]. In the eclipse phase, 1-2 weeks after infection, the T/F virus is freely replicating and spreading from the initial site of infection to the many tissues and organs that provide the sites for replication. At that stage viremia is still undetectable, and neither immune response nor symptoms of infection are yet visible. The next phase, also called acute or primary infection phase, 2-4 weeks after infection, is characterized by relatively high levels of viremia (up to 107 or more copies of viral RNA/mL of blood) and large fractions of infected CD4+ T cells in blood and lymph nodes. The high levels of viremia most likely result from the absence of the early immune response and the generation, as part of the host response, of large numbers of activated CD4+ T cells, thus providing extra targets for viral replication [145]. The rapid increase in HIV replication follows a striking induction of inflammatory cytokines and chemokines, which is in complete contrast to the minimum initial response to other chronic viral infections such as hepatitis B or hepatitis C [99][146].
–
The acute phase is often, but not always accompanied by “flu-like” symptoms including fever, sore throat, lymphadenopathy, and rash [147]. Around the time of peak viremia, the immune response begins to appear, both humoral (antibodies response against all viral antigens) and cellular (CD8+ T-cell response against HIV-1 antigens expressed on infected cells) [148]. The CD8 mediated killing of productively HIV-infected cells and the potent adaptive immune response to HIV, both select for the emergence of mutations in key viral epitopes which often leads to immune escape and so called highly diverse HIV quasispecies [99][149][150]. In certain cases, overrepresentation of specific HLA class I alleles (e.g. HLA-B27) is associated with an effective immune response, characterized by HIV-specific T cells with high avidity, polyfunctionality, and capacity to proliferate against both the immunodominant epitopes (e.g. for HLA-B27 the B27-KK10 epitope (KRWIILGLNK at positions 263-272) in p24 Gag) and escape variants thereof [151][152][153]. HLA alleles HLA-B57 and HLA-B5801 also exert strong selection pressure on the virus and are thus associated with long-term HIV control [154]. Interestingly, although HLA-B57 is correlated with slow progression to disease following HIV-1 infection, B-57 heterozygotes display a wide spectrum of outcomes, including rapid progression, viremic slow progression, and elite control [155].
–
However, only about 3% of the general population possess these specific HLA class I alleles, while in nearly all individuals a progressive exhaustion of HIV-specific CD8+ T cells occurs. This is characterized by an upregulation of the gene programmed death 1 (PD-1) expression on both total and HIV-specific CD8+ T cells and a loss of effector function. In addition, it was found that PD-1 expression levels correlate significantly with viral load and with the reduced capacity for cytokine production and proliferation of HIV-specific CD8+ T cells [156]. In contrast, CD8+ T-cells that see HLA B27/B57 cells in long-term nonprogressors do not get exhausted which helps these individuals to control the disease [157]. Similar studies in SIV infected rhesus macaques revealed that expression of the MHC class I alleles Mamu-B*08 and Mamu-B*17 correlates with viral control [158][159].
–
Autologous neutralizing antibodies arise roughly 3 months after infection [160]. The typical course of infection is characterized by the development of autologous antibody responses capable of neutralizing virus, selection of escape variants, development (after a delay) of new responses capable of neutralizing the new escape variants, and selection of new escape variants, with the host always playing catch-up [148][161]. Broadly neutralizing antibodies, which can neutralize many HIV-1 subtypes, are produced by about 20% of patients [162]. In addition, 2-4% of these patients have even greater serum neutralizing activity that inactivates most HIV-1 strains from different clades [163]. These antibodies are characterized by a high frequency of somatic mutations that often take years to develop [164]. However, as described above broadly neutralizing antibodies do not usually provide benefit to the patient because of the development of viral escape mutants. Nevertheless, antibodies also mediate important effector functions next to neutralization like antibody dependent cellular cytotoxicity (ADCC) [165][166], antibody-dependent cell-mediated virus inhibition (ADCVI) [167][168] or antibody-mediated phagocytosis [169][170]. Antibody effector functions mediated through Fc binding are thought to be one possible mechanism mediating protection from HIV-1 infection in humans in the recent Thai RV144 vaccine efficacy trial [121]. The production of broadly neutralizing antibodies by use of new immunogen design strategies is a major focus of vaccine research [171][172][173]. At the end of the acute phase the viral load decreases sharply, 100-fold or more, a result, which is established largely by innate and adaptive immune responses as well as exhaustion of activated target cells (i.e. transient decline in the CD4+ T cell number/mL blood) [145].
–
The chronic infection, or “clinical latency” (1-20 years after infection) is characterized by a constant or slowly increasing level of viremia (1-1×105 copies/mL), also called the “set point”, and steady, near normal (around 1,000 cells/mL) or gradually falling levels of CD4+ T cells. Usually no symptoms are shown during that phase making infected people unaware of their status [145]. Despite the term “latency,” the viral infection is far from latent, with large numbers of CD4+ T cells becoming infected and dying every day. Finally, the number of CD4+ T cells declines to the point (around 200 cells/mL) at which immune control of adventitious infectious agents can no longer be maintained, and opportunistic infections begin to appear [145]. Infections attributable to organisms such as Pneumocystis jirovecii, mycobacteria, cytomegalovirus, Toxoplasma gondii, and Cryptococcus as well as the occurrence of malignancies related to viral pathogens such as non-Hodgkins lymphoma and Kaposi’s sarcoma are common [174][175][176]. Nonetheless, the profound immune deficiency also affects humoral defenses, placing infected persons at increased risk for infection with pathogens like Streptococcus pneumoniae [176][177]. Control of the HIV-1 infection itself is also lost, and the level of viremia rises during the AIDS phase, culminating in death of the infected patient. Indeed, untreated HIV-1 infection is one of the most uniformly lethal infectious diseases known, with a mortality rate well over 95% [145].
–
Despite profound immune deficiency, there is evidence of profound immune activation in HIV infection as T lymphocytes, B-lymphocytes, and antigen-presenting cells of the innate immune system have phenotypic and functional evidence of activation [178][179]. In acute infection, a massive increase of cytokines, called cytokine storm, is a characteristic of HIV-1 infection [146] and the levels of some of these cytokines are predictive of the rate of CD4+ T-cell loss and of T-cell activation levels [180][181]. For example, CD8+ T cells often express high levels of activation markers such as CD38 and HLA-DR, which correlate with the viral load in non-controllers. Moreover, markers linked to immune senescence such as CD57 [182] and immune exhaustion such as PD-1 [156][183] are also elevated, and cells expressing each of these markers have demonstrable impairments in response to T cell receptor stimulation. Plasma levels of the IFN-inducible protein-10 (IP-10) during acute infection are predictive of rapid disease progression [181], while the frequency of CD8+ CD38+ cells is a valuable predictor of disease outcome in HIV infection and correlates well with the viral load [184]. Likewise, high levels of inflammatory and coagulation markers predict morbidity and mortality in treated HIV infection. One of the hallmarks of immune activation in HIV infection is the progressive depletion of circulating T cells by activation-induced cellular turnover [185][186][187]. This increased cellular turnover is seen in both CD4 and CD8 T-cell populations [187] and is especially remarkable among central memory cells in both humans and in SIV-infected macaques [188][189]. Activated cycling CD4+ T cells are more susceptible to productive HIV infection [190] [191] and also tend to die ex vivo, likely as a result of apoptosis [192].
–
Several potential drivers have been postulated to account for systemic immune activation in progressive HIV and SIV infection including the virus itself, which can drive activation of innate immune receptors such as TLR 7 and 8 through poly(U)-rich sequences in its genome [193][194] as well as possibly through activation of other innate immune receptors by capsid proteins [195] or viral DNAs [196]. A partial decrease in the abnormal cytokine profile is observed after the administration of highly active ART, which might contribute to lower levels of HIV replication and restoration of CD4 T cell counts [197]. Some level of T-cell activation in HIV infection might be also induced by the interaction of either HIV specific peptides or peptides from opportunistic microbes (such as cytomegalovirus and other herpes viruses), that have been permitted to replicate more effectively in the setting of HIV-related immune deficiency, with toll-like receptors (TCRs) [198]. It is also possible that some level of immune activation in HIV and pathogenic SIV infection is related to homeostatic mechanisms, that is, a need to replenish lymphocyte populations at effector sites of potential microbial invasion [199]. Additionally, there is increasing evidence that in HIV and in pathogenic SIV infection, early damage to mucosal CD4 T-cell defenses permits increased translocation of microbial products from the gut to the systemic circulation [200] and these microbial products can drive T-cell and innate immune cell activation [200][201].
–
In contrast, natural SIV hosts like sooty mangabeys, African green monkeys and mandrills share many features of HIV infection of humans; however, they usually do not develop immunodeficiency [202]. Interestingly, both innate and adaptive immune activation are observed during the acute phase of SIV infection of natural hosts [203][204] including strong upregulation of type I interferon–stimulated genes in both peripheral blood and lymph nodes [205] as well as type 1 interferon (IFN-1) production by plasmacytoid DCs in lymph nodes [206]. However, marked differences in the levels of immune activation between natural and non-natural HIV and SIV hosts are observed after the transition from the acute to the chronic phase of infection. Natural, nonprogressive SIV infections represent an evolutionary adaptation that allows a peaceful coexistence of primate lentiviruses and the host immune system. However, this adaptation does not result in reduced viral replication but, rather, involves phenotypic changes to CD4+ T cell subsets, limited immune activation and preserved mucosal immunity, all of which contribute to the avoidance of disease progression and, possibly, to the reduction of vertical SIV transmission [202].
–
High levels of circulating plasma IFN-1 in early HIV infection but not chronic HIV infection can suppress HIV replication and promote maturation and differentiation of antigen presenting cells such as DCs, monocytes, and macrophages [207]. In several studies of a cohort of HIV-exposed seronegative (HESN) commercial sex workers from Nairobi, Kenya, it could be demonstrated that qualitative differences in CD4+ T cell responses and HIV-specific CD4+ and CD8+ T cells, as well as genetic factors such as enrichment of certain HLA alleles and haplotypes near the IFN regulatory factor 1 gene have been associated with protection [208][209][210][211][212][213]. Furthermore it was shown that these individuals produce lower levels of proinflammatory cytokines at baseline than HIV-negative control subjects. Moreover, CD4+ T cells of these HESN commercial sex workers have a characteristically lower level of expression of genes and systems crucial for HIV replication, such as the T cell receptor pathway and previously identified HIV dependency factors [214]. This apparent lowered activation results in “immune quiescence,” which may contribute to host resistance to HIV [214]. Finally, data from two other groups have also shown an association between reduced T cell activation and decreased HIV susceptibility. Studies of highly exposed MSM showed that HESN individuals had lower levels of CD45RO+ memory T cells and a lower percentage of activated CD4+ T cells than HIV-susceptible control subjects [215]. Similarly, a study of HESN commercial sex workers from Côte d’Ivoire demonstrated lower expression of the activation marker CD69 in memory subsets of CD4+ and CD8+ T cells after alloimmune stimulation [216].
TREATMENT AND CURABILITY
HIV infection has developed from a fatal into a manageable chronic disease with life expectancy, in some instances, estimated to be near that of the general population [217]. Treatment with ART is life-long as it only suppresses the replication of HIV but does not eradicate or cure the infection [218]. Stopping ART results in HIV viral load rebound, progressive CD4+ T-cell count decline, and clinical disease progression [219]. One of the primary aims of ART treatment is to maintain health by preventing clinical disease progression at low cost of drug toxicity [220]. This is achieved by inhibiting viral replication by antivirals, resulting in long-term suppression of plasma viral load [221]. Treatment success is defined as maintaining plasma viral load at an undetectable level (<50 copies/mL) and by reconstitution of the immune system (Figure 5B) [222]. A secondary aim is prevention of HIV transmission by ‘treatment as prevention’ as described earlier [223]. Unfortunately, there is only limited evidence available from randomized trials to guide the decision to start therapy in naıve individuals. However, a recent study investigated the rate of new infections during administration of PrEP in a clinical practice setting. Despite high rates of sexually transmitted infections among PrEP users and reported decreases in condom use in a subset, no new HIV infections in this population were reported [224].
–
 | Figure 5: The course of untreated HIV infection and changes after antiretroviral therapy. In untreated HIV infection the blood CD4 T cell count progressively declines over the course of infection (A). After initiation of antiretroviral therapy the HIV RNA copy numbers significantly decrease below detection limit followed by recovery of CD4 T cells, which can vary notably between individuals (B). Adapted from [99]. |
Based on evidence from observational studies, current clinical guidelines all recommend that ART should be initiated if the CD4+ count is lower than 350 cells/mm3 or if an opportunistic infection has been diagnosed [225]. In contrast, clear evidence for starting ART early at higher CD4+ T cell counts is more ambiguous and recommendations differ [226][227][228]. Plausible arguments in favor of starting ART early include the likelihood of long-term clinical benefit, reduced risk of sexual HIV transmission and HSV-2 infection, concurrent treatment of HBV infection, improved tolerability of ART, reducing the latent reservoir size, and minimizing HIV-induced immune system damage [227]. Potential arguments against starting ART at higher CD4+ counts are the absence of evidence for clinical benefit to the individual, the potential for harm from ART and the risk of inducing HIV drug resistance resulting in the lifetime limiting of treatment options [228]. However, to date two studies — the TEMPRANO ANRS 12136 study [229] and the Strategic Timing of Antiretroviral Treatment (START) study [230] — provide important additional evidence to support early ART initiation by demonstrating its clinical benefits in asymptomatic patients at an early stage, when CD4+ cell counts are above 500 cells/mm3. The TEMPRANO study, involving 2056 patients in Ivory Coast, showed that early ART initiation (CD4+ cell count of ≥500 cells/mm3) was associated with a 44% lower risk of death or severe HIV-related illness than was ART initiated according to prevailing WHO criteria [231]. The START study, involving 4685 patients at 215 sites in 35 countries, showed that the risk of death, a serious AIDS-related event, or a serious non–AIDS-related event was 57% lower among those treated early than among those treated when the CD4+ cell count decreased to 350 cells/mm3 [231]. Patients initiating ART early in the START and TEMPRANO trials had viral suppression rates exceeding 95% and 80%, respectively. In both trials, a reduced rate of TB after early ART, as compared with deferred ART, was one of the most important contributors to the overall benefits [231]. The main barrier to starting early ART is late diagnosis [232]. For example, the majority of people in whom HIV is diagnosed each year in the UK have a CD4+ count lower than 350 cells/mm3 at time of testing, thus strategies are required to reduce late diagnosis [233]. Currently, more than 25 single or combination HIV drugs that block HIV replication at many steps in the virus lifecycle are approved by the U.S. Food and Drug Administration (FDA) for treatment of HIV infection. The drugs are grouped into six inhibitor classes: 1) Non-nucleoside reverse transcriptase inhibitors, 2) Nucleoside reverse transcriptase inhibitors, 3) Protease inhibitors, 4) Fusion inhibitors, 5) Entry inhibitors, and 6) Integrase inhibitors. Recommended ART regimens are less toxic, more effective, have a lower pill burden, and are dosed less frequently than the initial protease inhibitor-based regimens [234].
–
Therapy-naïve patients in high-income countries usually start a standard ART regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and either a ritonavir-boosted protease inhibitor, a non-nucleoside reverse transcriptase inhibitor (NNRTI) or an integrase inhibitor. For low-income and middle-income countries, WHO recommends a public health approach using ART with standardized first-line (NNRTI plus dual NRTIs) and second-line (ritonavir-boosted protease inhibitor plus dual NRTIs) regimens, and restricted monitoring for both efficacy and toxic effects [235]. Similar virological outcomes have been reported in a study that compared a public health approach with individualized approaches to ART in a high-income versus a low-income country. However, the switching rate for toxic effects in the high-income country was higher than in the low-income country [236]. After initiation of antiretroviral therapy, the plasma viral load decreases to concentrations below the lower limit of detection (< 50 copies/mL) in most people, usually within 3 months [237]. By contrast, the recovery of CD4 T cells in individuals on antiretroviral therapy is variable [238]. In one study the responses to ART at 6 months in low-income countries, showed that 56% of patients had a successful virological and CD4 response, 19% a virological response without a CD4 response, and 15% a CD4 response without a virological response. Individuals with impaired CD4 T-cell recovery despite virological suppression, which is associated with several risk factors, are at increased risk of adverse outcomes, including serious non-AIDS events [239][240]. Early mortality rates after initiation of antiretroviral therapy are much higher in resource-limited settings than in high-income countries, however with increasing duration on ART, mortality in HIV-infected patients on treatment in a middle-income locale declines rapidly to levels approaching those in high-income settings [241]. Successfully treated HIV-positive individuals have a near-normal life expectancy (other than people who inject drugs). Additionally, patients who achieve a normal CD4+ cell count and undetectable viral load on ART can significantly improve their life expectancy [242]. Antiretroviral therapy taken in the presence of continuing viral replication will result in the selection of sub-populations of HIV with mutations conferring virologic failure and drug resistance. Sub-optimum adherence is the major factor associated with the development of resistance [243]. Antiretroviral drugs differ in their ability to select for resistant mutations. Many factors determine the relative rate of resistance selection with different drugs and drug combinations. This is reflected in the “genetic barrier” to resistance, which refers to the number of mutations that must occur within a given target in order for resistance to be present against a particular drug [244]. Interactions between mutations, the effects of individual resistance mutations on viral replication capacity, and viral fitness all influence mutational pathways and the overall impact of resistance mutations on viral phenotype [244]. Several different mechanisms through which HIV-1 escapes from drug pressure have been described; these mechanisms differ from one drug class to another and can even differ between drugs of the same class. Also the number of mutations required for resistance to occur varies from drug to drug [244]. For instance, some drugs like the NNRTIs efavirenz and nevirapine or the integrase inhibitor raltegravir as well as the combination medicines emtricitabine and lamivudine rapidly select for one mutation conferring high-level resistance, whereas most other antiretrovirals select for resistance mutations slowly and need several resistant mutations before loss of drug efficacy [99]. Transmission of drug resistant virus strains is an emerging phenomenon with important clinical and public health implications. Prevalence estimates of transmission of drug-resistant HIV were found highest in North America (12.9%), followed by Europe (10.9%), Latin America (6.3%, Africa (4.7%), and Asia (4.2%) [245]. Transmissions of NRTI and NNRTI resistant viruses are the most common [244]. Remarkably, some HIV subtypes have a higher propensity to develop certain drug resistant mutations compared with others. For example, individuals infected with clade C have a higher incidence (70-87%) of nevirapine resistance (K103N, Y181C) compared to individuals with subtype A (42%). In addition, several studies found higher rates of the K65R mutation in clade C infected individuals treated with NRTIs compared to clade B infected individuals [246]. ART resistance selection studies revealed that the K65R mutation accumulated faster under TFV pressure compared to subtype B [247]. However, K65R is less frequent in subtype A than in all other subtypes [248]. Further selection studies have shown that a V106M mutation commonly develops in subtype C viruses following drug pressure with nevirapine or efavirenz, unlike the V106A mutation that is more commonly selected in subtype B. The clinical relevance of this mutation has been confirmed in recent years with several studies showing that V106M is frequently seen in non-B subtypes (i.e subtype C and CRF01_AE) after therapy with efavirenz or nevirapine [249]. In general, the effect of HIV-1 subtype diversity has not limited the overall benefit of ART, however, there are subtype differences in the type and preference of pathways of resistance with some mutations emerging almost exclusively in some non-B subtypes [246].
–
Although ART inhibits HIV replication and prevents disease progression, it does not eliminate the virus completely from infected patients, predominantly because of the presence of latently infected resting memory CD4+ T cells [250]. These latently infected cells contain viral DNA within their chromosomes but usually express little or no viral RNA and no viral proteins, thus rendering them beyond the reach of ART and essentially invisible to the immune system. However, upon stimulation these cells can produce infectious virus and rekindle virus replication if ART is discontinued [250]. In a recent study HIV sequences from resting CD4+ T cells from patients that were treated with ART during the acute phase of infection (within 3 months of HIV infection) were compared with those obtained from patients who initiated therapy during the chronic phase of infection. The analysis of the data revealed that known CTL escape variants were rare in acute phase-treated patients, whereas nearly all of the sequences from patients treated during the chronic phase harbored CTL escape mutations [251]. Defective HIV genomes tend to accumulate in CD4+ cells over the course of infection, indicating that most HIV DNA present in resting CD4+ T cells is defective rather than latent. Interestingly, the authors also demonstrated that in contrast to what is seen in individuals treated early in infection, replication-competent HIV induced from latently infected cells from patients treated in the chronic phase also bear a large number of CTL resistance mutations [251]. These data suggest that unless ART is initiated very early in the course of infection, the latent reservoir becomes populated almost exclusively with variants resistant to dominant CTL responses. Further, efforts directed toward stimulating a broader CTL response might be necessary to kill cells induced to express latent virus as well as therapeutic interventions such as, genetically engineered CTLs that are pre-programmed with T cell receptors specific for these alternative HIV epitopes, anti-HIV envelope immunotoxins, or broadly neutralizing antibodies that might also be very effective in this regard [250].
–
The fact that new CTL-resistant viruses largely replace the wild-type virus in the latent reservoir is of great interest as it indicates a more adaptable role of the latent reservoir, at least in the early stages of infection. This implies that the reservoir could be substantially depleted if the natural rate of elimination of latently infected cells in untreated infection could be maintained while preventing the formation anew of latency with ART, which is essentially the goal of activation-elimination approaches [250]. Indeed, several methods for purging the latent reservoir have been discussed. One strategy that is being actively investigated is an activation-elimination approach in which the host cell is induced to express viral proteins, allowing it to be killed by viral cytopathic effects or by the host immune response [252]. Various exogenous stimuli, including suberoylanilide hydroxamic acid, are currently being studied in efforts to safely and effectively activate latent HIV. However, some of these stimuli induce only low levels of virus expression, which might not be sufficient to kill the infected cell without a robust and effective immune response or other therapeutic intervention [253][254]. Other strategies (reviewed in [255]) for a functional cure besides enhancing specific immunity include 1) full or partial replacement of the immune system through genetic modifications, 2) shock and kill and 3) render HIV permanently silent. For example, the outcome of the “Berlin patient” who underwent an allogeneic stem transplant from a donor who was homozygous for the CCR5Δ32 deletion has been widely reported. In addition, Gene therapy with the aim to reduce CCR5 expression on T-cells, thus rendering them more resistant to HIV infection is currently being explored [255].
MOLECULAR MECHANISM OF INFECTION
The initial phase of the viral replication cycle begins with the adhesion of virus to the host cell and ends with the fusion of the cell and viral membranes with subsequent delivery of the viral core into the cytoplasm [256]. The complex series of protein–protein interactions that ultimately results in virus infection can be divided into several phases (Figure 6): First, virions must bind to the target cell, by either the viral envelope (Env) protein or through host cell membrane proteins incorporated into the virion [257]. Attachment can be either relatively nonspecific (e.g. Env interacting with sugar groups or lectin-like domains present on cell-surface receptors such as heparan sulfate or galactosylceramide) or more specific (e.g. interactions between Env and α4β7 integrin –the gut-homing receptor- or pattern recognition receptors such as DC–specific intercellular adhesion molecular 3-grabbing non-integrin (DC-SIGN)) [257]. HIV attachment to the host cell via any of these factors likely brings Env into close proximity with the host receptor CD4 and subsequently one of the coreceptors, thus increasing the efficiency of infection. However, attachment factors are not essential, and although they enhance infection in vitro, their physiological role in vivo remains unclear [256].
–
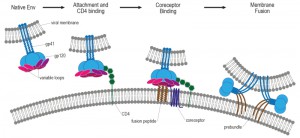 | Figure 6: Working model of HIV-1 entry. HIV entry is initiated by attachment of gp120 to CD4, which induces a conformational change in gp120. Following engagement of coreceptor, gp120 undergoes further conformational changes that allow for the insertion of the gp41 fusion peptide into the host membrane. The formation of the six-helix bundle brings the host and viral membranes into close proximity and creates a fusion pore, allowing entry of the HIV capsid into the host cell. Adapted from [258]. |
The second step of virus entry and absolutely required for infection involves engagement of Env with its primary host receptor, CD4 [258], which is a member of the immunoglobulin superfamily that normally functions to enhance T-cell receptor mediated signaling. Env is a heavily glyco-sylated trimer of gp120 and gp41 heterodimers and it is the sole target for HIV broadly neutralizing antibodies [259][260]. The host derived N-linked glycans of the HIV Env are crucial for correct protein folding as well as viral infectivity and modulating interactions with the host immune system [261]. The gp120 glycoprotein subunit is responsible for receptor binding [262] and is composed of five variable loops (V1–V5), named for their relative genetic heterogeneity, and five relatively conserved domains (C1–C5) [263]. The first four variable regions form surface-exposed loops composed by disulfide bonds at their bases, with the exception of V5 [264]. The variable surface exposed loops on gp120 play critical roles in immune evasion and coreceptor binding. Especially, the V3 loop is the principal determinant of chemokine receptor specificity [265]. Binding to the host cell CD4 receptor is mediated through the CD4 binding site (CD4bs) on gp120 and causes rearrangements of V1/V2 and subsequently V3. Additionally, CD4 binding leads to formation of the bridging sheet, a four- β strand structure comprised of two double-stranded β sheets that are spatially separated in the unliganded state [256].
–
The bridging sheet and the repositioned V3 loop are critical elements for coreceptor binding in the next step of virus entry [266]. Coreceptor binding is widely thought to be the final trigger that activates membrane fusion [267]. The relevance of viral coreceptors for subsequent HIV in-fection in vivo was demonstrated by the identification of a 32-base-pair deletion in CCR5, termed CCR5-Δ32, which is characterized by a premature stop codon in the second extracellular loop of CCR5 and subsequent retention of the mutant protein in the endoplasmic reticulum. The frequency of the CCR5-Δ32 allele in European Caucasians is around 10%, whereas it is absent in Africans and East Asians [268]. Individuals who are CCR5-Δ32 homozygous or CCR5-Δ32/Δ32 have non-functional CCR5 receptors, resulting in profound resistance to HIV infection. However, individuals with homozygosity for CCR5-Δ32 are very rare (about 1% of Caucasians). In contrast, heterozygous individuals, who possess one copy of CCR5-Δ32 and one copy of CCR5-wildtype, are more frequent (20%) and have altered chemokine receptor activity. There is strong evidence that heterozygosity for CCR5-Δ32 provides partial protection against sexual transmission of HIV infection both from male-to-male as well as from male-to-female [268]. HIV strains that use the chemokine receptor CCR5 are called R5 HIV, those that use CXCR4 are termed X4 HIV, and viruses that can use both coreceptors are called R5X4 HIV [269]. Although both R5 and X4 HIV-1 variants are present in body fluids (semen, blood, cervicovaginal and rectal secretions) and despite high levels of CXCR4 expression on circulating HIV target cells, only R5 viruses are transmitted between individuals and dominate early stages of HIV disease [270].
–
A fourth step of virus entry involves trafficking to specific entry sites where viruses encounter a milieu that provides for productive entry and membrane fusion occurs [271]. A series of studies [271][272][273] have shown that a number of viruses hijack cellular transport pathways to reach specific destinations that are either needed for infection or that make entry more efficient, and that HIV might likewise use the host cell machinery to reach sites where membrane fusion can occur [256]. Some viruses, including HIV, have been shown to attach to the plasma membrane and “surf” along the cell surface, moving from distal sites of attachment to more proximal regions of the cell body where virus entry occurs [256][271][274]. A recent study proposed that complete HIV fusion occurs in endosomes, as viral fusion with the plasma membrane does not progress beyond the lipid-mixing step. It was further shown that HIV virions underwent receptor-mediated internalization long before endosomal fusion, thus minimizing the surface exposure of conserved viral epitopes during fusion and reducing the efficacy of inhibitors targeting these epitopes [275].
–
The final step of virus entry is membrane fusion mediated by the engagement of Env with the CD4 receptor and coreceptor (i.e. CXCR4 or CCR5). Coreceptor binding induces a conformational change in Env, which causes the fusion peptide (FP) of gp41 to insert into the host cell membrane [276]. Simultaneously, a coiled-coil forms comprising three adjacent amino-terminal helical regions (NHR) of gp41, the grooves of which form high affinity binding sites for the carboxy-terminal helical region (CHR) to bind in an antiparallel orientation. The result of the NHR-CHR interaction is an energetically stable 6-helix bundle (6HB), that pulls together the apposing membranes of the host cell and virus to consummate the fusion reaction [276], and results in the formation of a fusion pore [277]. However, it is likely that several Env trimers are needed to form a fusion pore [267]. In summary, coreceptor binding unlocks the potential energy of the gp41 fusion complex resulting in 6HB formation, opening, and stabilization of the membrane fusion pore, and subsequent delivery of the viral contents into the host cell cytoplasm.
–
HIV can disseminate between CD4+ T cells either via cell-free diffusion-limited viral spread, or by directed cell-cell transfer using virally induced structures termed virological synapses (i.e. organized contact areas, which concentrate cellular entry receptors and virions) [278]. In vitro, HIV spreads very efficiently, if not preferentially, by cell-cell contacts from infected to non-infected cells via 1) formation of virological synapses, 2) transient cell-cell contacts, and 3) longer-range intercellular interactions including nanotubes and filopodia [279][280]. Virus transmission through these mechanisms has been proven to be more efficient and rapid than infection by cell free viruses [281][282] thus supporting the notion that cell-cell transmission might be a relevant if not dominant mode of virus transmission in vivo [279].
–
Advances in electron microscopy have enabled three-dimensional-structural studies of the virological synapse that have shed light on this mechanism of infection [283]. DCs, which are professional antigen presenting cells often found scavenging the periphery, produce membranous protrusions capable of trapping virions in a surface-accessible but protected compartment [284]. Each DC can bind up to several hundred virions [285] most likely via a C-type lectin such as DC-SIGN [284][286]. It remains unclear if these protrusions occur before or after virion binding and whether it is Env induced [256]. When CD4+ T cells contact DCs, they extend filopodia, enriched for CD4 and coreceptor, into the invaginated DC compartments that containing bound virions. Together, the efficient binding of HIV, relocalization to the point of CD4+ T-cell contact, and the recruitment of the requisite HIV entry receptors promote HIV entrance at the infectious synapse [285][287]. However, so far the relative contribution of cell-cell and cell-free virus transmission in acquisition of HIV infection and viral dissemination during human infection remains undefined.
–
In conclusion, defeating the HIV/AIDS pandemic has proven a challenging task. Nonetheless, significant advances in our understanding of the virus and the disease it causes have been transformed into improvements in the life expectancy for those affected. While a preventive vaccine or a cure has not been achieved to date, other approaches such as on-demand-PrEP appear to reduce the rate of transmission when the individuals involved adhere to a program. Our current knowledge of the virus’ biology has provided us with glimmers of solutions, and the risks derived from the improper application of therapies. Likewise, the importance of education and socio-economic factors in this endeavor cannot be overstated. Our survey of the literature shows that the continuous cooperation among all parties in the struggle against the HIV/AIDS pandemic has been vital in the advances made to date.
References
- P.M. Sharp, and B.H. Hahn, "Origins of HIV and the AIDS Pandemic", Cold Spring Harbor Perspectives in Medicine, vol. 1, pp. a006841-a006841, 2011. http://dx.doi.org/10.1101/cshperspect.a006841
- K. Hymes, J. Greene, A. Marcus, D. William, T. Cheung, N. Prose, H. Ballard, and L. Laubenstein, "KAPOSI'S SARCOMA IN HOMOSEXUAL MEN—A REPORT OF EIGHT CASES", The Lancet, vol. 318, pp. 598-600, 1981. http://dx.doi.org/10.1016/s0140-6736(81)92740-9
- H. Masur, M.A. Michelis, J.B. Greene, I. Onorato, R.A. Vande Stouwe, R.S. Holzman, G. Wormser, L. Brettman, M. Lange, H.W. Murray, and S. Cunningham-Rundles, "An Outbreak of Community-AcquiredPneumocystis cariniiPneumonia", New England Journal of Medicine, vol. 305, pp. 1431-1438, 1981. http://dx.doi.org/10.1056/NEJM198112103052402
- S. Broder, and R.C. Gallo, "A Pathogenic Retrovirus (HTLV-III) Linked to AIDS", New England Journal of Medicine, vol. 311, pp. 1292-1297, 1984. http://dx.doi.org/10.1056/NEJM198411153112006
- P. Piot, H. Taelman, K. Bila Minlangu, N. Mbendi, K. Ndangi, K. Kalambayi, C. Bridts, T. Quinn, F. Feinsod, O. Wobin, P. Mazebo, W. Stevens, S. Mitchell, and J. Mccormick, "ACQUIRED IMMUNODEFICIENCY SYNDROME IN A HETEROSEXUAL POPULATION IN ZAIRE", The Lancet, vol. 324, pp. 65-69, 1984. http://dx.doi.org/10.1016/s0140-6736(84)90241-1
- P. Van De Perre, P. Lepage, P. Kestelyn, A. Hekker, D. Rouvroy, J. Bogaerts, J. Kayihigi, J. Butzler, and N. Clumeck, "ACQUIRED IMMUNODEFICIENCY SYNDROME IN RWANDA", The Lancet, vol. 324, pp. 62-65, 1984. http://dx.doi.org/10.1016/S0140-6736(84)90240-X
- B.H. Hahn, G.M. Shaw, K.M. De, . Cock, and P.M. Sharp, "AIDS as a Zoonosis: Scientific and Public Health Implications", Science, vol. 287, pp. 607-614, 2000. http://dx.doi.org/10.1126/science.287.5453.607
- N.R. Faria, A. Rambaut, M.A. Suchard, G. Baele, T. Bedford, M.J. Ward, A.J. Tatem, J.D. Sousa, N. Arinaminpathy, J. Pépin, D. Posada, M. Peeters, O.G. Pybus, and P. Lemey, "The early spread and epidemic ignition of HIV-1 in human populations", Science, vol. 346, pp. 56-61, 2014. http://dx.doi.org/10.1126/science.1256739
- T. Mourez, F. Simon, and J. Plantier, "Non-M Variants of Human Immunodeficiency Virus Type 1", Clinical Microbiology Reviews, vol. 26, pp. 448-461, 2013. http://dx.doi.org/10.1128/CMR.00012-13
- M. Peeters, M. D'Arc, and E. Delaporte, "Origin and diversity of human retroviruses.", AIDS reviews, 2014. http://www.ncbi.nlm.nih.gov/pubmed/24584106
- S.B. Lloyd, S.J. Kent, and W.R. Winnall, "The High Cost of Fidelity", AIDS Research and Human Retroviruses, vol. 30, pp. 8-16, 2014. http://dx.doi.org/10.1089/AID.2013.0153
- W.H. Li, M. Tanimura, and P.M. Sharp, "Rates and dates of divergence between AIDS virus nucleotide sequences.", Molecular biology and evolution, 1988. http://www.ncbi.nlm.nih.gov/pubmed/3405075
- D. Smith, D. Richman, and S. Little, "HIV Superinfection", The Journal of Infectious Diseases, vol. 192, pp. 438-444, 2005. http://dx.doi.org/10.1086/431682
- V. Simon, D.D. Ho, and Q. Abdool Karim, "HIV/AIDS epidemiology, pathogenesis, prevention, and treatment", The Lancet, vol. 368, pp. 489-504, 2006. http://dx.doi.org/10.1016/S0140-6736(06)69157-5
- . UNAIDS, "AIDS by the numbers.", Available at: http://www.unaids.org/en/resources/documents/2015/AIDS_by_the_numbers_2015. Accessed 15.12.2015, 2015.
- O. Shisana, L. SImbayi, K. Zuma, S. Jooste, N. Zungu, D. Labadarios, and D. Onoya, "South African National HIV Prevalence, Incidence and Behaviour Survey, 2012.", Cape Town HSRC Press, 2014.
- . WHO, "HIV/AIDS.", Available at: http://www.who.int/hiv/data/epi_core_july2015.png. Accessed: 15.12.2015, 2015.
- . UNAIDS, "The Gap report.", Available at: http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report. Accessed: 18.12.2015, 2014.
- Q.A. Karim, A.B. Kharsany, J.A. Frohlich, L. Werner, M. Mashego, M. Mlotshwa, B.T. Madlala, F. Ntombela, and S.S. Abdool Karim, "Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa", International Journal of Epidemiology, vol. 40, pp. 922-930, 2010. http://dx.doi.org/10.1093/ije/dyq176
- R.C. Dellar, S. Dlamini, and Q.A. Karim, "Adolescent girls and young women: key populations for HIV epidemic control", Journal of the International AIDS Society, vol. 18, 2015. http://dx.doi.org/10.7448/IAS.18.2.19408
- L. Bekker, L. Johnson, M. Wallace, and S. Hosek, "Building our youth for the future", Journal of the International AIDS Society, vol. 18, 2015. http://dx.doi.org/10.7448/IAS.18.2.20027
- . UNAIDS, "HIV in Asia and the Pacific.", Available at: http://www.unaids.org/en/resources/documents/2013/20131119_HIV-Asia-Pacific. Accessed: 18.12.2015, 2013.
- A. Cowell, S.V. Shenoi, T.C. Kyriakides, G. Friedland, and L.A. Barakat, "Trends in hospital deaths among human immunodeficiency virus–infected patients during the antiretroviral therapy era, 1995 to 2011", Journal of Hospital Medicine, vol. 10, pp. 608-614, 2015. http://dx.doi.org/10.1002/jhm.2409
- V.A. Triant, "Cardiovascular Disease and HIV Infection", Current HIV/AIDS Reports, vol. 10, pp. 199-206, 2013. http://dx.doi.org/10.1007/s11904-013-0168-6
- S. Saravanan, "Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India", World Journal of Gastroenterology, vol. 13, pp. 5015, 2007. http://dx.doi.org/10.3748/WJG.v13.i37.5015
- J. Khalsa, and A. Elkashef, "Interventions for HIV and Hepatitis C Virus Infections in Recreational Drug Users", Clinical Infectious Diseases, vol. 50, pp. 1505-1511, 2010. http://dx.doi.org/10.1086/652447
- P. Sonnenberg, J. Glynn, K. Fielding, J. Murray, P. Godfrey‐Faussett, and S. Shearer, "How Soon after Infection with HIV Does the Risk of Tuberculosis Start to Increase? A Retrospective Cohort Study in South African Gold Miners", The Journal of Infectious Diseases, vol. 191, pp. 150-158, 2005. http://dx.doi.org/10.1086/426827
- C.B. Holmes, R. Wood, M. Badri, S. Zilber, B. Wang, G. Maartens, H. Zheng, Z. Lu, K.A. Freedberg, and E. Losina, "CD4 Decline and Incidence of Opportunistic Infections in Cape Town, South Africa", JAIDS Journal of Acquired Immune Deficiency Syndromes, vol. 42, pp. 464-469, 2006. http://dx.doi.org/10.1097/01.qai.0000225729.79610.b7
- . UNAIDS, "Fact Sheet.", Available at: http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet. Accessed: 22.12.2015, 2015.
- I. Chiu, A. Yaniv, J.E. Dahlberg, A. Gazit, S.F. Skuntz, S.R. Tronick, and S.A. Aaronson, "Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses", Nature, vol. 317, pp. 366-368, 1985. http://dx.doi.org/10.1038/317366a0
- J.F. Hutchinson, "The Biology and Evolution of HIV", Annual Review of Anthropology, vol. 30, pp. 85-108, 2001. http://dx.doi.org/10.1146/annurev.anthro.30.1.85
- E.O. Freed, "HIV-1 replication.", Somatic cell and molecular genetics, 2001. http://www.ncbi.nlm.nih.gov/pubmed/12465460
- J.A. Jablonski, A.L. Amelio, M. Giacca, and M. Caputi, "The transcriptional transactivator Tat selectively regulates viral splicing", Nucleic Acids Research, vol. 38, pp. 1249-1260, 2009. http://dx.doi.org/10.1093/nar/gkp1105
- A. Cimarelli, and J. Darlix, "HIV-1 Reverse Transcription", Methods in Molecular Biology, pp. 55-70, 2014. http://dx.doi.org/10.1007/978-1-62703-670-2_6
- M. Chamanian, K. Purzycka, P. Wille, J. Ha, D. McDonald, Y. Gao, S. Le Grice, and E. Arts, "A cis-Acting Element in Retroviral Genomic RNA Links Gag-Pol Ribosomal Frameshifting to Selective Viral RNA Encapsidation", Cell Host & Microbe, vol. 13, pp. 181-192, 2013. http://dx.doi.org/10.1016/j.chom.2013.01.007
- J. Coffin, S. Hughes, and H. Varmus, "The Interactions of Retroviruses and their Hosts. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses.", Cold Spring Harbor, New York, 1997.
- R. Craigie, and F.D. Bushman, "HIV DNA Integration", Cold Spring Harbor Perspectives in Medicine, vol. 2, pp. a006890-a006890, 2012. http://dx.doi.org/10.1101/cshperspect.a006890
- T. Hewson, N. Lone, M. Moore, and S. Howie, "Interactions of HIV‐1 with antigen‐presenting cells", Immunology & Cell Biology, vol. 77, pp. 289-303, 1999. http://dx.doi.org/10.1046/j.1440-1711.1999.00833.x
- R. Swanstrom, and J. Coffin, "HIV-1 Pathogenesis: The Virus", Cold Spring Harbor Perspectives in Medicine, vol. 2, pp. a007443-a007443, 2012. http://dx.doi.org/10.1101/cshperspect.a007443
- J. Campo, M. Perea, J. Del Romero, J. Cano, V. Hernando, and A. Bascones, "Oral transmission of HIV, reality or fiction? An update", Oral Diseases, vol. 12, pp. 219-228, 2006. http://dx.doi.org/10.1111/j.1601-0825.2005.01187.x
- A.E. Petroll, C.B. Hare, and S.D. Pinkerton, "The Essentials of HIV", Journal of Infusion Nursing, vol. 31, pp. 228-235, 2008. http://dx.doi.org/10.1097/01.NAN.0000326831.82526.c4
- L. Morison, "The global epidemiology of HIV/AIDS", British Medical Bulletin, vol. 58, pp. 7-18, 2001. http://dx.doi.org/10.1093/bmb/58.1.7
- P. Patel, C.B. Borkowf, J.T. Brooks, A. Lasry, A. Lansky, and J. Mermin, "Estimating per-act HIV transmission risk", AIDS, vol. 28, pp. 1509-1519, 2014. http://dx.doi.org/10.1097/QAD.0000000000000298
- A.E. Grulich, and I. Zablotska, "Commentary: Probability of HIV transmission through anal intercourse", International Journal of Epidemiology, vol. 39, pp. 1064-1065, 2010. http://dx.doi.org/10.1093/ije/dyq101
- M. Boily, R.F. Baggaley, L. Wang, B. Masse, R.G. White, R.J. Hayes, and M. Alary, "Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies", The Lancet Infectious Diseases, vol. 9, pp. 118-129, 2009. http://dx.doi.org/10.1016/S1473-3099(09)70021-0
- B.F. Keele, and J.D. Estes, "Barriers to mucosal transmission of immunodeficiency viruses", Blood, vol. 118, pp. 839-846, 2011. http://dx.doi.org/10.1182/blood-2010-12-325860
- B.L. Shacklett, "Mucosal Immunity to HIV-1", Encyclopedia of AIDS, pp. 1-13, 2014. http://dx.doi.org/10.1007/978-1-4614-9610-6_197-1
- T.C. Quinn, M.J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M.O. Meehan, T. Lutalo, and R.H. Gray, "Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1", New England Journal of Medicine, vol. 342, pp. 921-929, 2000. http://dx.doi.org/10.1056/NEJM200003303421303
- J.M. Baeten, E. Kahle, J.R. Lingappa, R.W. Coombs, S. Delany-Moretlwe, E. Nakku-Joloba, N.R. Mugo, A. Wald, L. Corey, D. Donnell, M.S. Campbell, J.I. Mullins, and C. Celum, "Genital HIV-1 RNA Predicts Risk of Heterosexual HIV-1 Transmission", Science Translational Medicine, vol. 3, 2011. http://dx.doi.org/10.1126/scitranslmed.3001888
- R.M. Ribeiro, L. Qin, L.L. Chavez, D. Li, S.G. Self, and A.S. Perelson, "Estimation of the Initial Viral Growth Rate and Basic Reproductive Number during Acute HIV-1 Infection", Journal of Virology, vol. 84, pp. 6096-6102, 2010. http://dx.doi.org/10.1128/JVI.00127-10
- J.P. Hughes, J.M. Baeten, J.R. Lingappa, A.S. Magaret, A. Wald, G. de Bruyn, J. Kiarie, M. Inambao, W. Kilembe, C. Farquhar, C. Celum, and . , "Determinants of Per-Coital-Act HIV-1 Infectivity Among African HIV-1–Serodiscordant Couples", The Journal of Infectious Diseases, vol. 205, pp. 358-365, 2012. http://dx.doi.org/10.1093/infdis/jir747
- M.S. Cohen, "Preventing Sexual Transmission of HIV", Clinical Infectious Diseases, vol. 45, pp. S287-S292, 2007. http://dx.doi.org/10.1086/522552
- M. Wawer, R. Gray, N. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire‐Mangen, M. Meehan, and T. Quinn, "Rates of HIV‐1 Transmission per Coital Act, by Stage of HIV‐1 Infection, in Rakai, Uganda", The Journal of Infectious Diseases, vol. 191, pp. 1403-1409, 2005. http://dx.doi.org/10.1086/429411
- S.R. Galvin, and M.S. Cohen, "The role of sexually transmitted diseases in HIV transmission", Nature Reviews Microbiology, vol. 2, pp. 33-42, 2004. http://dx.doi.org/10.1038/nrmicro794
- J. Reis Machado, M.V. da Silva, C.L. Cavellani, M. Antônia dos Reis, M.L.G.D.R. Monteiro, V.D.P.A. Teixeira, and R. Rosa Miranda Corrêa, "Mucosal Immunity in the Female Genital Tract, HIV/AIDS", BioMed Research International, vol. 2014, pp. 1-20, 2014. http://dx.doi.org/10.1155/2014/350195
- J. Sheffield, G. Wendel, Jr., D. McIntire, and M. Norgard, "Effect of Genital Ulcer Disease on HIV‐1 Coreceptor Expression in the Female Genital Tract", The Journal of Infectious Diseases, vol. 196, pp. 1509-1516, 2007. http://dx.doi.org/10.1086/522518
- J.M. Marrazzo, K.K. Thomas, and K. Ringwood, "A behavioural intervention to reduce persistence of bacterial vaginosis among women who report sex with women: results of a randomised trial", Sexually Transmitted Infections, vol. 87, pp. 399-405, 2011. http://dx.doi.org/10.1136/sti.2011.049213
- P. Mirmonsef, L. Krass, A. Landay, and G. T. Spear, "The Role of Bacterial Vaginosis and Trichomonas in HIV Transmission Across The Female Genital Tract", Current HIV Research, vol. 10, pp. 202-210, 2012. http://dx.doi.org/10.2174/157016212800618165
- M.S. Cohen, I.F. Hoffman, R.A. Royce, P. Kazembe, J.R. Dyer, C.C. Daly, D. Zimba, P.L. Vernazza, M. Maida, S.A. Fiscus, and J.J. Eron, "Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1", The Lancet, vol. 349, pp. 1868-1873, 1997. http://dx.doi.org/10.1016/s0140-6736(97)02190-9
- S.C. Shafir, F.J. Sorvillo, and L. Smith, "Current Issues and Considerations Regarding Trichomoniasis and Human Immunodeficiency Virus in African-Americans", Clinical Microbiology Reviews, vol. 22, pp. 37-45, 2009. http://dx.doi.org/10.1128/CMR.00002-08
- J. Lama, and V. Planelles, "Host factors influencing susceptibility to HIV infection and AIDS progression", Retrovirology, vol. 4, pp. 52, 2007. http://dx.doi.org/10.1186/1742-4690-4-52
- G. Ramjee, and B. Daniels, "Women and HIV in Sub-Saharan Africa", AIDS Research and Therapy, vol. 10, pp. 30, 2013. http://dx.doi.org/10.1186/1742-6405-10-30
- T. Hirbod, X. Kong, G. Kigozi, A. Ndyanabo, D. Serwadda, J.L. Prodger, A.A. Tobian, F. Nalugoda, M.J. Wawer, K. Shahabi, O.L. Rojas, J.L. Gommerman, K. Broliden, R. Kaul, and R.H. Gray, "HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men", PLoS Pathogens, vol. 10, pp. e1004416, 2014. http://dx.doi.org/10.1371/journal.ppat.1004416
- P.M. Leclerc, N. Dubois-Colas, and M. Garenne, "Hormonal contraception and HIV prevalence in four African countries", Contraception, vol. 77, pp. 371-376, 2008. http://dx.doi.org/10.1016/j.contraception.2008.01.012
- D. Watson-Jones, K. Baisley, H.A. Weiss, C. Tanton, J. Changalucha, D. Everett, T. Chirwa, D. Ross, T. Clayton, and R. Hayes, "Risk factors for HIV incidence in women participating in an HSV suppressive treatment trial in Tanzania", AIDS, vol. 23, pp. 415-422, 2009. http://dx.doi.org/10.1097/QAD.0b013e32831ef523
- Z. Hel, E. Stringer, and J. Mestecky, "Sex Steroid Hormones, Hormonal Contraception, and the Immunobiology of Human Immunodeficiency Virus-1 Infection", Endocrine Reviews, vol. 31, pp. 79-97, 2009. http://dx.doi.org/10.1210/er.2009-0018
- D. Wilkinson, G. Ramjee, M. Tholandi, and G.W. Rutherford, "Nonoxynol-9 for preventing vaginal acquisition of HIV infection by women from men", Cochrane Database of Systematic Reviews, vol. 2012, 2002. http://dx.doi.org/10.1002/14651858.CD003936
- D. Wilkinson, M. Tholandi, G. Ramjee, and G.W. Rutherford, "Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women", The Lancet Infectious Diseases, vol. 2, pp. 613-617, 2002. http://dx.doi.org/10.1016/s1473-3099(02)00396-1
- M. Laga, and P. Piot, "Prevention of sexual transmission of HIV", AIDS, vol. 26, pp. 1223-1229, 2012. http://dx.doi.org/10.1097/QAD.0b013e32835462b8
- J.M. Marrazzo, and W. Cates, "Interventions to Prevent Sexually Transmitted Infections, Including HIV Infection", Clinical Infectious Diseases, vol. 53, pp. S64-S78, 2011. http://dx.doi.org/10.1093/cid/cir695
- L.W. Chang, D. Serwadda, T.C. Quinn, M.J. Wawer, R.H. Gray, and S.J. Reynolds, "Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects", The Lancet Infectious Diseases, vol. 13, pp. 65-76, 2013. http://dx.doi.org/10.1016/S1473-3099(12)70273-6
- M.S. Cohen, Y.Q. Chen, M. McCauley, T. Gamble, M.C. Hosseinipour, N. Kumarasamy, J.G. Hakim, J. Kumwenda, B. Grinsztejn, J.H. Pilotto, S.V. Godbole, S. Mehendale, S. Chariyalertsak, B.R. Santos, K.H. Mayer, I.F. Hoffman, S.H. Eshleman, E. Piwowar-Manning, L. Wang, J. Makhema, L.A. Mills, G. de Bruyn, I. Sanne, J. Eron, J. Gallant, D. Havlir, S. Swindells, H. Ribaudo, V. Elharrar, D. Burns, T.E. Taha, K. Nielsen-Saines, D. Celentano, M. Essex, and T.R. Fleming, "Prevention of HIV-1 Infection with Early Antiretroviral Therapy", New England Journal of Medicine, vol. 365, pp. 493-505, 2011. http://dx.doi.org/10.1056/NEJMoa1105243
- F. Tanser, T. Bärnighausen, E. Grapsa, J. Zaidi, and M. Newell, "High Coverage of ART Associated with Decline in Risk of HIV Acquisition in Rural KwaZulu-Natal, South Africa", Science, vol. 339, pp. 966-971, 2013. http://dx.doi.org/10.1126/science.1228160
- "Erratum", Tropical Medicine & International Health, vol. 19, pp. 1525-1525, 2014. http://dx.doi.org/10.1111/tmi.12420
- K. Jean, D. Gabillard, R. Moh, C. Danel, R. Fassassi, A. Desgrées-du-Loû, S. Eholié, F. Lert, X. Anglaret, and R. Dray-Spira, "Effect of Early Antiretroviral Therapy on Sexual Behaviors and HIV-1 Transmission Risk Among Adults With Diverse Heterosexual Partnership Statuses in Côte d'Ivoire", The Journal of Infectious Diseases, vol. 209, pp. 431-440, 2013. http://dx.doi.org/10.1093/infdis/jit470
- N.D. Labhardt, M. Motlomelo, B. Cerutti, K. Pfeiffer, M. Kamele, M.A. Hobbins, and J. Ehmer, "Home-Based Versus Mobile Clinic HIV Testing and Counseling in Rural Lesotho: A Cluster-Randomized Trial", PLoS Medicine, vol. 11, pp. e1001768, 2014. http://dx.doi.org/10.1371/journal.pmed.1001768
- A.B. Suthar, N. Ford, P.J. Bachanas, V.J. Wong, J.S. Rajan, A.K. Saltzman, O. Ajose, A.O. Fakoya, R.M. Granich, E.K. Negussie, and R.C. Baggaley, "Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches", PLoS Medicine, vol. 10, pp. e1001496, 2013. http://dx.doi.org/10.1371/journal.pmed.1001496
- M. Sharma, R. Ying, G. Tarr, and R. Barnabas, "Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa", Nature, vol. 528, pp. S77-S85, 2015. http://dx.doi.org/10.1038/nature16044
- B.E. Nichols, C.A.B. Boucher, and D.A.M.C. van de Vijver, "HIV testing and antiretroviral treatment strategies for prevention of HIV infection: impact on antiretroviral drug resistance", Journal of Internal Medicine, vol. 270, pp. 532-549, 2011. http://dx.doi.org/10.1111/j.1365-2796.2011.02456.x
- S. Tully, M. Cojocaru, and C.T. Bauch, "Sexual behavior, risk perception and HIV transmission can respond to HIV antiviral drugs and vaccines through multiple pathways", Scientific Reports, vol. 5, 2015. http://dx.doi.org/10.1038/srep15411
- A. Medley, C. Garcia-Moreno, S. McGill, and S. Maman, "Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes.", Bulletin of the World Health Organization, 2004. http://www.ncbi.nlm.nih.gov/pubmed/15259260
- R.M. Grant, J.R. Lama, P.L. Anderson, V. McMahan, A.Y. Liu, L. Vargas, P. Goicochea, M. Casapía, J.V. Guanira-Carranza, M.E. Ramirez-Cardich, O. Montoya-Herrera, T. Fernández, V.G. Veloso, S.P. Buchbinder, S. Chariyalertsak, M. Schechter, L. Bekker, K.H. Mayer, E.G. Kallás, K.R. Amico, K. Mulligan, L.R. Bushman, R.J. Hance, C. Ganoza, P. Defechereux, B. Postle, F. Wang, J.J. McConnell, J. Zheng, J. Lee, J.F. Rooney, H.S. Jaffe, A.I. Martinez, D.N. Burns, and D.V. Glidden, "Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men", New England Journal of Medicine, vol. 363, pp. 2587-2599, 2010. http://dx.doi.org/10.1056/NEJMoa1011205
- M.C. Thigpen, P.M. Kebaabetswe, L.A. Paxton, D.K. Smith, C.E. Rose, T.M. Segolodi, F.L. Henderson, S.R. Pathak, F.A. Soud, K.L. Chillag, R. Mutanhaurwa, L.I. Chirwa, M. Kasonde, D. Abebe, E. Buliva, R.J. Gvetadze, S. Johnson, T. Sukalac, V.T. Thomas, C. Hart, J.A. Johnson, C.K. Malotte, C.W. Hendrix, and J.T. Brooks, "Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana", New England Journal of Medicine, vol. 367, pp. 423-434, 2012. http://dx.doi.org/10.1056/NEJMoa1110711
- K. Choopanya, M. Martin, P. Suntharasamai, U. Sangkum, P.A. Mock, M. Leethochawalit, S. Chiamwongpaet, P. Kitisin, P. Natrujirote, S. Kittimunkong, R. Chuachoowong, R.J. Gvetadze, J.M. McNicholl, L.A. Paxton, M.E. Curlin, C.W. Hendrix, and S. Vanichseni, "Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial", The Lancet, vol. 381, pp. 2083-2090, 2013. http://dx.doi.org/10.1016/S0140-6736(13)61127-7
- J.M. Baeten, D. Donnell, P. Ndase, N.R. Mugo, J.D. Campbell, J. Wangisi, J.W. Tappero, E.A. Bukusi, C.R. Cohen, E. Katabira, A. Ronald, E. Tumwesigye, E. Were, K.H. Fife, J. Kiarie, C. Farquhar, G. John-Stewart, A. Kakia, J. Odoyo, A. Mucunguzi, E. Nakku-Joloba, R. Twesigye, K. Ngure, C. Apaka, H. Tamooh, F. Gabona, A. Mujugira, D. Panteleeff, K.K. Thomas, L. Kidoguchi, M. Krows, J. Revall, S. Morrison, H. Haugen, M. Emmanuel-Ogier, L. Ondrejcek, R.W. Coombs, L. Frenkel, C. Hendrix, N.N. Bumpus, D. Bangsberg, J.E. Haberer, W.S. Stevens, J.R. Lingappa, and C. Celum, "Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women", New England Journal of Medicine, vol. 367, pp. 399-410, 2012. http://dx.doi.org/10.1056/NEJMoa1108524
- J. Molina, C. Pintado, C. Gatey, D. Ponscarme, P. Charbonneau, B. Loze, W. Rozenbaum, and C. Delaugerre, "Challenges and opportunities for oral pre-exposure prophylaxis in the prevention of HIV infection: where are we in Europe?", BMC Medicine, vol. 11, 2013. http://dx.doi.org/10.1186/1741-7015-11-186
- J. Molina, C. Capitant, B. Spire, G. Pialoux, L. Cotte, I. Charreau, C. Tremblay, J. Le Gall, E. Cua, A. Pasquet, F. Raffi, C. Pintado, C. Chidiac, J. Chas, P. Charbonneau, C. Delaugerre, M. Suzan-Monti, B. Loze, J. Fonsart, G. Peytavin, A. Cheret, J. Timsit, G. Girard, N. Lorente, M. Préau, J.F. Rooney, M.A. Wainberg, D. Thompson, W. Rozenbaum, V. Doré, L. Marchand, M. Simon, N. Etien, J. Aboulker, L. Meyer, and J. Delfraissy, "On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection", New England Journal of Medicine, vol. 373, pp. 2237-2246, 2015. http://dx.doi.org/10.1056/NEJMoa1506273
- R.H. Gray, G. Kigozi, D. Serwadda, F. Makumbi, S. Watya, F. Nalugoda, N. Kiwanuka, L.H. Moulton, M.A. Chaudhary, M.Z. Chen, N.K. Sewankambo, F. Wabwire-Mangen, M.C. Bacon, C.F. Williams, P. Opendi, S.J. Reynolds, O. Laeyendecker, T.C. Quinn, and M.J. Wawer, "Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial", The Lancet, vol. 369, pp. 657-666, 2007. http://dx.doi.org/10.1016/S0140-6736(07)60313-4
- R.C. Bailey, S. Moses, C.B. Parker, K. Agot, I. Maclean, J.N. Krieger, C.F. Williams, R.T. Campbell, and J.O. Ndinya-Achola, "Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial", The Lancet, vol. 369, pp. 643-656, 2007. http://dx.doi.org/10.1016/S0140-6736(07)60312-2
- R.G. Wamai, B.J. Morris, S.A. Bailis, D. Sokal, J.D. Klausner, R. Appleton, N. Sewankambo, D.A. Cooper, J. Bongaarts, G. de Bruyn, A.D. Wodak, and J. Banerjee, "Male circumcision for HIV prevention: current evidence and implementation in sub‐Saharan Africa", Journal of the International AIDS Society, vol. 14, pp. 49-49, 2011. http://dx.doi.org/10.1186/1758-2652-14-49
- A.A. Tobian, S. Kacker, and T.C. Quinn, "Male Circumcision: A Globally Relevant but Under-Utilized Method for the Prevention of HIV and Other Sexually Transmitted Infections", Annual Review of Medicine, vol. 65, pp. 293-306, 2014. http://dx.doi.org/10.1146/annurev-med-092412-090539
- C. Dobard, S. Sharma, A. Martin, C. Pau, A. Holder, Z. Kuklenyik, J. Lipscomb, D.L. Hanson, J. Smith, F.J. Novembre, J.G. García-Lerma, and W. Heneine, "Durable Protection from Vaginal Simian-Human Immunodeficiency Virus Infection in Macaques by Tenofovir Gel and Its Relationship to Drug Levels in Tissue", Journal of Virology, vol. 86, pp. 718-725, 2012. http://dx.doi.org/10.1128/JVI.05842-11
- Q. Abdool Karim, S.S. Abdool Karim, J.A. Frohlich, A.C. Grobler, C. Baxter, L.E. Mansoor, A.B.M. Kharsany, S. Sibeko, K.P. Mlisana, Z. Omar, T.N. Gengiah, S. Maarschalk, N. Arulappan, M. Mlotshwa, L. Morris, D. Taylor, and . , "Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women", Science, vol. 329, pp. 1168-1174, 2010. http://dx.doi.org/10.1126/science.1193748
- C.W. Dobard, S. Sharma, M. Cong, R. West, N. Makarova, A. Holder, C. Pau, D.L. Hanson, F.J. Novembre, J.G. Garcia-Lerma, and W. Heneine, "Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques", Retrovirology, vol. 12, 2015. http://dx.doi.org/10.1186/s12977-015-0195-z
- H. Rees, D. Baron, C. Lombard, G. Gray, L. Myer, R. Panchia, J. Schwartz, and G. Doncel, "FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women (abstract 26LB).", Program and abstracts of the 2015 Conference on Retroviruses and Opportunistic Infections (CROI) CROI, Seattle, 2015.
- K.H. Mayer, "NextGen HIV prevention: new possibilities and questions", The Lancet, vol. 387, pp. 1036-1038, 2016. http://dx.doi.org/10.1016/S0140-6736(16)00655-3
- B. Devlin, J. Nuttall, S. Wilder, C. Woodsong, and Z. Rosenberg, "Development of dapivirine vaginal ring for HIV prevention", Antiviral Research, vol. 100, pp. S3-S8, 2013. http://dx.doi.org/10.1016/j.antiviral.2013.09.025
- J.M. Baeten, T. Palanee-Phillips, E.R. Brown, K. Schwartz, L.E. Soto-Torres, V. Govender, N.M. Mgodi, F. Matovu Kiweewa, G. Nair, F. Mhlanga, S. Siva, L. Bekker, N. Jeenarain, Z. Gaffoor, F. Martinson, B. Makanani, A. Pather, L. Naidoo, M. Husnik, B.A. Richardson, U.M. Parikh, J.W. Mellors, M.A. Marzinke, C.W. Hendrix, A. van der Straten, G. Ramjee, Z.M. Chirenje, C. Nakabiito, T.E. Taha, J. Jones, A. Mayo, R. Scheckter, J. Berthiaume, E. Livant, C. Jacobson, P. Ndase, R. White, K. Patterson, D. Germuga, B. Galaska, K. Bunge, D. Singh, D.W. Szydlo, E.T. Montgomery, B.S. Mensch, K. Torjesen, C.I. Grossman, N. Chakhtoura, A. Nel, Z. Rosenberg, I. McGowan, and S. Hillier, "Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women", New England Journal of Medicine, vol. 375, pp. 2121-2132, 2016. http://dx.doi.org/10.1056/NEJMoa1506110
- G. Maartens, C. Celum, and S.R. Lewin, "HIV infection: epidemiology, pathogenesis, treatment, and prevention", The Lancet, vol. 384, pp. 258-271, 2014. http://dx.doi.org/10.1016/S0140-6736(14)60164-1
- R. V. Barnabas, and C. Celum, "Infectious Co-Factors in HIV-1 Transmission Herpes Simplex Virus Type-2 and HIV-1: New Insights and Interventions", Current HIV Research, vol. 10, pp. 228-237, 2012. http://dx.doi.org/10.2174/157016212800618156
- A.M. Buchanan, and C.K. Cunningham, "Advances and Failures in Preventing Perinatal Human Immunodeficiency Virus Infection", Clinical Microbiology Reviews, vol. 22, pp. 493-507, 2009. http://dx.doi.org/10.1128/CMR.00054-08
- M.L. Newell, "Prevention of mother-to-child transmission of HIV: challenges for the current decade.", Bulletin of the World Health Organization, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11799446
- C. Chasela, Y.Q. Chen, S. Fiscus, I. Hoffman, A. Young, M. Valentine, L. Emel, T.E. Taha, R.L. Goldenberg, and J.S. Read, "Risk Factors for Late Postnatal Transmission of Human Immunodeficiency Virus Type 1 in Sub-Saharan Africa", Pediatric Infectious Disease Journal, vol. 27, pp. 251-256, 2008. http://dx.doi.org/10.1097/INF.0b013e31815b4960
- . , "Late Postnatal Transmission of HIV‐1 in Breast‐Fed Children: An Individual Patient Data Meta‐Analysis", The Journal of Infectious Diseases, vol. 189, pp. 2154-2166, 2004. http://dx.doi.org/10.1086/420834
- H.M. Coovadia, N.C. Rollins, R.M. Bland, K. Little, A. Coutsoudis, M.L. Bennish, and M. Newell, "Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study", The Lancet, vol. 369, pp. 1107-1116, 2007. http://dx.doi.org/10.1016/S0140-6736(07)60283-9
- G. John-Stewart, D. Mbori-Ngacha, R. Ekpini, E.N. Janoff, J. Nkengasong, J.S. Read, P. Van de Perre, M. Newell, and . , "Breast-feeding and Transmission of HIV-1.", Journal of acquired immune deficiency syndromes (1999), 2004. http://www.ncbi.nlm.nih.gov/pubmed/14722454
- D. Dunn, M. Newell, A. Ades, and C. Peckham, "Risk of human immunodeficiency virus type 1 transmission through breastfeeding", The Lancet, vol. 340, pp. 585-588, 1992. http://dx.doi.org/10.1016/0140-6736(92)92115-v
- C.L. Townsend, L. Byrne, M. Cortina-Borja, C. Thorne, A. de Ruiter, H. Lyall, G.P. Taylor, C.S. Peckham, and P.A. Tookey, "Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011", AIDS, vol. 28, pp. 1049-1057, 2014. http://dx.doi.org/10.1097/QAD.0000000000000212
- G. John, R. Nduati, D. Mbori‐Ngacha, B. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. Ndinya‐Achola, and J. Kreiss, "Correlates of Mother‐to‐Child Human Immunodeficiency Virus Type 1 (HIV‐1) Transmission: Association with Maternal Plasma HIV‐1 RNA Load, Genital HIV‐1 DNA Shedding, and Breast Infections", The Journal of Infectious Diseases, vol. 183, pp. 206-212, 2001. http://dx.doi.org/10.1086/317918
- M.G. Fowler, A.P. Kourtis, J. Aizire, C. Onyango-Makumbi, and M. Bulterys, "Breastfeeding and Transmission of HIV-1: Epidemiology and Global Magnitude", Advances in Experimental Medicine and Biology, pp. 3-25, 2012. http://dx.doi.org/10.1007/978-1-4614-2251-8_1
- A.L. Drake, A. Wagner, B. Richardson, and G. John-Stewart, "Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis", PLoS Medicine, vol. 11, pp. e1001608, 2014. http://dx.doi.org/10.1371/journal.pmed.1001608
- G. Meintjes, G. Maartens, and O.B.O.T. Southern African HIV Clinicians Society, "Guidelines for antiretroviral therapy in adults", Southern African Journal of HIV Medicine, vol. 13, pp. 114-133, 2012. http://dx.doi.org/10.4102/sajhivmed.v13i3.125
- S.M. Hammer, M.E. Sobieszczyk, H. Janes, S.T. Karuna, M.J. Mulligan, D. Grove, B.A. Koblin, S.P. Buchbinder, M.C. Keefer, G.D. Tomaras, N. Frahm, J. Hural, C. Anude, B.S. Graham, M.E. Enama, E. Adams, E. DeJesus, R.M. Novak, I. Frank, C. Bentley, S. Ramirez, R. Fu, R.A. Koup, J.R. Mascola, G.J. Nabel, D.C. Montefiori, J. Kublin, M.J. McElrath, L. Corey, and P.B. Gilbert, "Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine", New England Journal of Medicine, vol. 369, pp. 2083-2092, 2013. http://dx.doi.org/10.1056/NEJMoa1310566
- . , "Placebo‐Controlled Phase 3 Trial of a Recombinant Glycoprotein 120 Vaccine to Prevent HIV‐1 Infection", The Journal of Infectious Diseases, vol. 191, pp. 654-665, 2005. http://dx.doi.org/10.1086/428404
- P. Pitisuttithum, P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. Tappero, K. Choopanya, and . , "Randomized, Double‐Blind, Placebo‐Controlled Efficacy Trial of a Bivalent Recombinant Glycoprotein 120 HIV‐1 Vaccine among Injection Drug Users in Bangkok, Thailand", The Journal of Infectious Diseases, vol. 194, pp. 1661-1671, 2006. http://dx.doi.org/10.1086/508748
- S.P. Buchbinder, D.V. Mehrotra, A. Duerr, D.W. Fitzgerald, R. Mogg, D. Li, P.B. Gilbert, J.R. Lama, M. Marmor, C. del Rio, M.J. McElrath, D.R. Casimiro, K.M. Gottesdiener, J.A. Chodakewitz, L. Corey, and M.N. Robertson, "Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial", The Lancet, vol. 372, pp. 1881-1893, 2008. http://dx.doi.org/10.1016/S0140-6736(08)61591-3
- M.J. McElrath, S.C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O.D. Defawe, D.K. Carter, J. Hural, R. Akondy, S.P. Buchbinder, M.N. Robertson, D.V. Mehrotra, S.G. Self, L. Corey, J.W. Shiver, and D.R. Casimiro, "HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case–cohort analysis", The Lancet, vol. 372, pp. 1894-1905, 2008. http://dx.doi.org/10.1016/S0140-6736(08)61592-5
- G.E. Gray, M. Allen, Z. Moodie, G. Churchyard, L. Bekker, M. Nchabeleng, K. Mlisana, B. Metch, G. de Bruyn, M.H. Latka, S. Roux, M. Mathebula, N. Naicker, C. Ducar, D.K. Carter, A. Puren, N. Eaton, M.J. McElrath, M. Robertson, L. Corey, and J.G. Kublin, "Safety and efficacy of the HVTN 503/Phambili Study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study", The Lancet Infectious Diseases, vol. 11, pp. 507-515, 2011. http://dx.doi.org/10.1016/S1473-3099(11)70098-6
- B.F. Haynes, P.B. Gilbert, M.J. McElrath, S. Zolla-Pazner, G.D. Tomaras, S.M. Alam, D.T. Evans, D.C. Montefiori, C. Karnasuta, R. Sutthent, H. Liao, A.L. DeVico, G.K. Lewis, C. Williams, A. Pinter, Y. Fong, H. Janes, A. DeCamp, Y. Huang, M. Rao, E. Billings, N. Karasavvas, M.L. Robb, V. Ngauy, M.S. de Souza, R. Paris, G. Ferrari, R.T. Bailer, K.A. Soderberg, C. Andrews, P.W. Berman, N. Frahm, S.C. De Rosa, M.D. Alpert, N.L. Yates, X. Shen, R.A. Koup, P. Pitisuttithum, J. Kaewkungwal, S. Nitayaphan, S. Rerks-Ngarm, N.L. Michael, and J.H. Kim, "Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial", New England Journal of Medicine, vol. 366, pp. 1275-1286, 2012. http://dx.doi.org/10.1056/NEJMoa1113425
- M. Vaccari, P. Poonam, and G. Franchini, "Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV", Expert Review of Vaccines, vol. 9, pp. 997-1005, 2010. http://dx.doi.org/10.1586/erv.10.104
- S. Rerks-Ngarm, P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J.G. McNeil, D.P. Francis, D. Stablein, D.L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M.L. Robb, N.L. Michael, P. Kunasol, and J.H. Kim, "Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand", New England Journal of Medicine, vol. 361, pp. 2209-2220, 2009. http://dx.doi.org/10.1056/NEJMoa0908492
-
H. Xu, X. Wang, and R.S. Veazey, "Mucosal immunology of
HIV infection", Immunological Reviews, vol. 254, pp. 10-33, 2013. http://dx.doi.org/10.1111/imr.12072 - R.J. Shattock, B.F. Haynes, B. Pulendran, J. Flores, J. Esparza, and . , "Improving Defences at the Portal of HIV Entry: Mucosal and Innate Immunity", PLoS Medicine, vol. 5, pp. e81, 2008. http://dx.doi.org/10.1371/journal.pmed.0050081
- S. Akira, S. Uematsu, and O. Takeuchi, "Pathogen Recognition and Innate Immunity", Cell, vol. 124, pp. 783-801, 2006. http://dx.doi.org/10.1016/j.cell.2006.02.015
- N.K. Saksena, B. Wang, L. Zhou, M. Soedjono, Y.S. Ho, and V. Conceicao, "HIV reservoirs in vivo and new strategies for possible eradication of HIV from the reservoir sites.", HIV/AIDS (Auckland, N.Z.), 2010. http://www.ncbi.nlm.nih.gov/pubmed/22096389
- P. Chen, B.K. Chen, A. Mosoian, T. Hays, M.J. Ross, P.E. Klotman, and M.E. Klotman, "Virological Synapses Allow HIV-1 Uptake and Gene Expression in Renal Tubular Epithelial Cells", Journal of the American Society of Nephrology, vol. 22, pp. 496-507, 2011. http://dx.doi.org/10.1681/ASN.2010040379
- C.J. Miller, and R.J. Shattock, "Target cells in vaginal HIV transmission", Microbes and Infection, vol. 5, pp. 59-67, 2003. http://dx.doi.org/10.1016/s1286-4579(02)00056-4
- S. Gupta, J.S. Gach, J.C. Becerra, T.B. Phan, J. Pudney, Z. Moldoveanu, S.B. Joseph, G. Landucci, M.J. Supnet, L. Ping, D. Corti, B. Moldt, Z. Hel, A. Lanzavecchia, R.M. Ruprecht, D.R. Burton, J. Mestecky, D.J. Anderson, and D.N. Forthal, "The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells", PLoS Pathogens, vol. 9, pp. e1003776, 2013. http://dx.doi.org/10.1371/journal.ppat.1003776
- M. Pope, and A.T. Haase, "Transmission, acute HIV-1 infection and the quest for strategies to prevent infection", Nature Medicine, vol. 9, pp. 847-852, 2003. http://dx.doi.org/10.1038/nm0703-847
- A.A. Lackner, and R.S. Veazey, "Current Concepts in AIDS Pathogenesis: Insights from the SIV/Macaque Model", Annual Review of Medicine, vol. 58, pp. 461-476, 2007. http://dx.doi.org/10.1146/annurev.med.58.082405.094316
- Z.F. Parker, S.S. Iyer, C.B. Wilen, N.F. Parrish, K.C. Chikere, F. Lee, C.A. Didigu, R. Berro, P.J. Klasse, B. Lee, J.P. Moore, G.M. Shaw, B.H. Hahn, and R.W. Doms, "Transmitted/Founder and Chronic HIV-1 Envelope Proteins Are Distinguished by Differential Utilization of CCR5", Journal of Virology, vol. 87, pp. 2401-2411, 2013. http://dx.doi.org/10.1128/JVI.02964-12
- B.F. Keele, E.E. Giorgi, J.F. Salazar-Gonzalez, J.M. Decker, K.T. Pham, M.G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J.L. Kirchherr, F. Gao, J.A. Anderson, L. Ping, R. Swanstrom, G.D. Tomaras, W.A. Blattner, P.A. Goepfert, J.M. Kilby, M.S. Saag, E.L. Delwart, M.P. Busch, M.S. Cohen, D.C. Montefiori, B.F. Haynes, B. Gaschen, G.S. Athreya, H.Y. Lee, N. Wood, C. Seoighe, A.S. Perelson, T. Bhattacharya, B.T. Korber, B.H. Hahn, and G.M. Shaw, "Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection", Proceedings of the National Academy of Sciences, vol. 105, pp. 7552-7557, 2008. http://dx.doi.org/10.1073/pnas.0802203105
- N.F. Parrish, F. Gao, H. Li, E.E. Giorgi, H.J. Barbian, E.H. Parrish, L. Zajic, S.S. Iyer, J.M. Decker, A. Kumar, B. Hora, A. Berg, F. Cai, J. Hopper, T.N. Denny, H. Ding, C. Ochsenbauer, J.C. Kappes, R.P. Galimidi, A.P. West, P.J. Bjorkman, C.B. Wilen, R.W. Doms, M. O’Brien, N. Bhardwaj, P. Borrow, B.F. Haynes, M. Muldoon, J.P. Theiler, B. Korber, G.M. Shaw, and B.H. Hahn, "Phenotypic properties of transmitted founder HIV-1", Proceedings of the National Academy of Sciences, vol. 110, pp. 6626-6633, 2013. http://dx.doi.org/10.1073/pnas.1304288110
- A.E. Fenton-May, O. Dibben, T. Emmerich, H. Ding, K. Pfafferott, M.M. Aasa-Chapman, P. Pellegrino, I. Williams, M.S. Cohen, F. Gao, G.M. Shaw, B.H. Hahn, C. Ochsenbauer, J.C. Kappes, and P. Borrow, "Relative resistance of HIV-1 founder viruses to control by interferon-alpha", Retrovirology, vol. 10, 2013. http://dx.doi.org/10.1186/1742-4690-10-146
- C. Ochsenbauer, T.G. Edmonds, H. Ding, B.F. Keele, J. Decker, M.G. Salazar, J.F. Salazar-Gonzalez, R. Shattock, B.F. Haynes, G.M. Shaw, B.H. Hahn, and J.C. Kappes, "Generation of Transmitted/Founder HIV-1 Infectious Molecular Clones and Characterization of Their Replication Capacity in CD4 T Lymphocytes and Monocyte-Derived Macrophages", Journal of Virology, vol. 86, pp. 2715-2728, 2012. http://dx.doi.org/10.1128/JVI.06157-11
- J.F. Salazar-Gonzalez, M.G. Salazar, B.F. Keele, G.H. Learn, E.E. Giorgi, H. Li, J.M. Decker, S. Wang, J. Baalwa, M.H. Kraus, N.F. Parrish, K.S. Shaw, M.B. Guffey, K.J. Bar, K.L. Davis, C. Ochsenbauer-Jambor, J.C. Kappes, M.S. Saag, M.S. Cohen, J. Mulenga, C.A. Derdeyn, S. Allen, E. Hunter, M. Markowitz, P. Hraber, A.S. Perelson, T. Bhattacharya, B.F. Haynes, B.T. Korber, B.H. Hahn, and G.M. Shaw, "Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection", Journal of Experimental Medicine, vol. 206, pp. 1273-1289, 2009. http://dx.doi.org/10.1084/jem.20090378
- M. Alexander, R. Lynch, J. Mulenga, S. Allen, C.A. Derdeyn, and E. Hunter, "Donor and Recipient Envs from Heterosexual Human Immunodeficiency Virus Subtype C Transmission Pairs Require High Receptor Levels for Entry", Journal of Virology, vol. 84, pp. 4100-4104, 2010. http://dx.doi.org/10.1128/JVI.02068-09
- J. Isaacman-Beck, E.A. Hermann, Y. Yi, S.J. Ratcliffe, J. Mulenga, S. Allen, E. Hunter, C.A. Derdeyn, and R.G. Collman, "Heterosexual Transmission of Human Immunodeficiency Virus Type 1 Subtype C: Macrophage Tropism, Alternative Coreceptor Use, and the Molecular Anatomy of CCR5 Utilization", Journal of Virology, vol. 83, pp. 8208-8220, 2009. http://dx.doi.org/10.1128/JVI.00296-09
- C.A. Derdeyn, J.M. Decker, F. Bibollet-Ruche, J.L. Mokili, M. Muldoon, S.A. Denham, M.L. Heil, F. Kasolo, R. Musonda, B.H. Hahn, G.M. Shaw, B.T. Korber, S. Allen, and E. Hunter, "Envelope-Constrained Neutralization-Sensitive HIV-1 After Heterosexual Transmission", Science, vol. 303, pp. 2019-2022, 2004. http://dx.doi.org/10.1126/science.1093137
- H. Zhang, M. Rola, J.T. West, D.C. Tully, P. Kubis, J. He, C. Kankasa, and C. Wood, "Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission", Virology, vol. 400, pp. 164-174, 2010. http://dx.doi.org/10.1016/j.virol.2009.12.019
- F. Nawaz, C. Cicala, D. Van Ryk, K.E. Block, K. Jelicic, J.P. McNally, O. Ogundare, M. Pascuccio, N. Patel, D. Wei, A.S. Fauci, and J. Arthos, "The Genotype of Early-Transmitting HIV gp120s Promotes α4β7 –Reactivity, Revealing α4β7+/CD4+ T cells As Key Targets in Mucosal Transmission", PLoS Pathogens, vol. 7, pp. e1001301, 2011. http://dx.doi.org/10.1371/journal.ppat.1001301
- B. Chohan, D. Lang, M. Sagar, B. Korber, L. Lavreys, B. Richardson, and J. Overbaugh, "Selection for Human Immunodeficiency Virus Type 1 Envelope Glycosylation Variants with Shorter V1-V2 Loop Sequences Occurs during Transmission of Certain Genetic Subtypes and May Impact Viral RNA Levels", Journal of Virology, vol. 79, pp. 6528-6531, 2005. http://dx.doi.org/10.1128/JVI.79.10.6528-6531.2005
- Y. Liu, M.E. Curlin, K. Diem, H. Zhao, A.K. Ghosh, H. Zhu, A.S. Woodward, J. Maenza, C.E. Stevens, J. Stekler, A.C. Collier, I. Genowati, W. Deng, R. Zioni, L. Corey, T. Zhu, and J.I. Mullins, "Env length and N-linked glycosylation following transmission of human immunodeficiency virus Type 1 subtype B viruses", Virology, vol. 374, pp. 229-233, 2008. http://dx.doi.org/10.1016/j.virol.2008.01.029
- C.B. Wilen, N.F. Parrish, J.M. Pfaff, J.M. Decker, E.A. Henning, H. Haim, J.E. Petersen, J.A. Wojcechowskyj, J. Sodroski, B.F. Haynes, D.C. Montefiori, J.C. Tilton, G.M. Shaw, B.H. Hahn, and R.W. Doms, "Phenotypic and Immunologic Comparison of Clade B Transmitted/Founder and Chronic HIV-1 Envelope Glycoproteins", Journal of Virology, vol. 85, pp. 8514-8527, 2011. http://dx.doi.org/10.1128/JVI.00736-11
- J. Coffin, and R. Swanstrom, "HIV Pathogenesis: Dynamics and Genetics of Viral Populations and Infected Cells", Cold Spring Harbor Perspectives in Medicine, vol. 3, pp. a012526-a012526, 2013. http://dx.doi.org/10.1101/cshperspect.a012526
- A.R. Stacey, P.J. Norris, L. Qin, E.A. Haygreen, E. Taylor, J. Heitman, M. Lebedeva, A. DeCamp, D. Li, D. Grove, S.G. Self, and P. Borrow, "Induction of a Striking Systemic Cytokine Cascade prior to Peak Viremia in Acute Human Immunodeficiency Virus Type 1 Infection, in Contrast to More Modest and Delayed Responses in Acute Hepatitis B and C Virus Infections", Journal of Virology, vol. 83, pp. 3719-3733, 2009. http://dx.doi.org/10.1128/JVI.01844-08
- J.M. Pincus, S.S. Crosby, E. Losina, E.R. King, C. LaBelle, and K.A. Freedberg, "Acute Human Immunodeficiency Virus Infection in Patients Presenting to an Urban Urgent Care Center", Clinical Infectious Diseases, vol. 37, pp. 1699-1704, 2003. http://dx.doi.org/10.1086/379772
- L.J. Picker, S.G. Hansen, and J.D. Lifson, "New Paradigms for HIV/AIDS Vaccine Development", Annual Review of Medicine, vol. 63, pp. 95-111, 2012. http://dx.doi.org/10.1146/annurev-med-042010-085643
- N. Goonetilleke, M.K. Liu, J.F. Salazar-Gonzalez, G. Ferrari, E. Giorgi, V.V. Ganusov, B.F. Keele, G.H. Learn, E.L. Turnbull, M.G. Salazar, K.J. Weinhold, S. Moore, . , N. Letvin, B.F. Haynes, M.S. Cohen, P. Hraber, T. Bhattacharya, P. Borrow, A.S. Perelson, B.H. Hahn, G.M. Shaw, B.T. Korber, and A.J. McMichael, "The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection", Journal of Experimental Medicine, vol. 206, pp. 1253-1272, 2009. http://dx.doi.org/10.1084/jem.20090365
- C.L. Althaus, and R.J. De Boer, "Dynamics of Immune Escape during HIV/SIV Infection", PLoS Computational Biology, vol. 4, pp. e1000103, 2008. http://dx.doi.org/10.1371/journal.pcbi.1000103
- K. Ladell, M. Hashimoto, M. Iglesias, P. Wilmann, J. McLaren, S. Gras, T. Chikata, N. Kuse, S. Fastenackels, E. Gostick, J. Bridgeman, V. Venturi, Z. Arkoub, H. Agut, D. van Bockel, J. Almeida, D. Douek, L. Meyer, A. Venet, M. Takiguchi, J. Rossjohn, D. Price, and V. Appay, "A Molecular Basis for the Control of Preimmune Escape Variants by HIV-Specific CD8+ T Cells", Immunity, vol. 38, pp. 425-436, 2013. http://dx.doi.org/10.1016/j.immuni.2012.11.021
- M. Altfeld, E.T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M.N. Johnston, N. Burgett, M.E. Swartz, A. Yang, G. Alter, X.G. Yu, A. Meier, J.K. Rockstroh, T.M. Allen, H. Jessen, E.S. Rosenberg, M. Carrington, and B.D. Walker, "HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8+ T Cell Response against HIV-1", PLoS Medicine, vol. 3, pp. e403, 2006. http://dx.doi.org/10.1371/journal.pmed.0030403
- A. Scherer, J. Frater, A. Oxenius, J. Agudelo, D.A. Price, H.F. Günthard, M. Barnardo, L. Perrin, B. Hirschel, R.E. Phillips, A.R. McLean, and . , "Quantifiable cytotoxic T lymphocyte responses and HLA-related risk of progression to AIDS", Proceedings of the National Academy of Sciences, vol. 101, pp. 12266-12270, 2004. http://dx.doi.org/10.1073/pnas.0404091101
- A.J. Leslie, K.J. Pfafferott, P. Chetty, R. Draenert, M.M. Addo, M. Feeney, Y. Tang, E.C. Holmes, T. Allen, J.G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S.A. Thomas, A.S. John, T.A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B.D. Walker, and P.J.R. Goulder, "HIV evolution: CTL escape mutation and reversion after transmission", Nature Medicine, vol. 10, pp. 282-289, 2004. http://dx.doi.org/10.1038/nm992
- C.A. Brennan, F.J. Ibarrondo, C.A. Sugar, M.A. Hausner, R. Shih, H.L. Ng, R. Detels, J.B. Margolick, C.R. Rinaldo, J. Phair, L.P. Jacobson, O.O. Yang, and B.D. Jamieson, "Early HLA-B*57-Restricted CD8+T Lymphocyte Responses Predict HIV-1 Disease Progression", Journal of Virology, vol. 86, pp. 10505-10516, 2012. http://dx.doi.org/10.1128/JVI.00102-12
- L. Trautmann, L. Janbazian, N. Chomont, E.A. Said, S. Gimmig, B. Bessette, M. Boulassel, E. Delwart, H. Sepulveda, R.S. Balderas, J. Routy, E.K. Haddad, and R. Sekaly, "Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction", Nature Medicine, vol. 12, pp. 1198-1202, 2006. http://dx.doi.org/10.1038/nm1482
- S. Elahi, W.L. Dinges, N. Lejarcegui, K.J. Laing, A.C. Collier, D.M. Koelle, M.J. McElrath, and H. Horton, "Protective HIV-specific CD8+ T cells evade Treg cell suppression", Nature Medicine, vol. 17, pp. 989-995, 2011. http://dx.doi.org/10.1038/nm.2422
- J.T. Loffredo, J. Maxwell, Y. Qi, C.E. Glidden, G.J. Borchardt, T. Soma, A.T. Bean, D.R. Beal, N.A. Wilson, W.M. Rehrauer, J.D. Lifson, M. Carrington, and D.I. Watkins, "Mamu-B*08-Positive Macaques Control Simian Immunodeficiency Virus Replication", Journal of Virology, vol. 81, pp. 8827-8832, 2007. http://dx.doi.org/10.1128/JVI.00895-07
- L.J. Yant, T.C. Friedrich, R.C. Johnson, G.E. May, N.J. Maness, A.M. Enz, J.D. Lifson, D.H. O'Connor, M. Carrington, and D.I. Watkins, "The High-Frequency Major Histocompatibility Complex Class I Allele Mamu-B * 17 Is Associated with Control of Simian Immunodeficiency Virus SIVmac239 Replication", Journal of Virology, vol. 80, pp. 5074-5077, 2006. http://dx.doi.org/10.1128/JVI.80.10.5074-5077.2006
- A.J. McMichael, P. Borrow, G.D. Tomaras, N. Goonetilleke, and B.F. Haynes, "The immune response during acute HIV-1 infection: clues for vaccine development", Nature Reviews Immunology, vol. 10, pp. 11-23, 2009. http://dx.doi.org/10.1038/nri2674
- D.D. Richman, T. Wrin, S.J. Little, and C.J. Petropoulos, "Rapid evolution of the neutralizing antibody response to HIV type 1 infection", Proceedings of the National Academy of Sciences, vol. 100, pp. 4144-4149, 2003. http://dx.doi.org/10.1073/pnas.0630530100
- J. Huang, B.H. Kang, M. Pancera, J.H. Lee, T. Tong, Y. Feng, H. Imamichi, I.S. Georgiev, G. Chuang, A. Druz, N.A. Doria-Rose, L. Laub, K. Sliepen, M.J. van Gils, A.T. de la Peña, R. Derking, P. Klasse, S.A. Migueles, R.T. Bailer, M. Alam, P. Pugach, B.F. Haynes, R.T. Wyatt, R.W. Sanders, J.M. Binley, A.B. Ward, J.R. Mascola, P.D. Kwong, and M. Connors, "Broad and potent HIV-1 neutralization by a human antibody that binds the gp41–gp120 interface", Nature, vol. 515, pp. 138-142, 2014. http://dx.doi.org/10.1038/nature13601
- M.D. Simek, W. Rida, F.H. Priddy, P. Pung, E. Carrow, D.S. Laufer, J.K. Lehrman, M. Boaz, T. Tarragona-Fiol, G. Miiro, J. Birungi, A. Pozniak, D.A. McPhee, O. Manigart, E. Karita, A. Inwoley, W. Jaoko, J. DeHovitz, L. Bekker, P. Pitisuttithum, R. Paris, L.M. Walker, P. Poignard, T. Wrin, P.E. Fast, D.R. Burton, and W.C. Koff, "Human Immunodeficiency Virus Type 1 Elite Neutralizers: Individuals with Broad and Potent Neutralizing Activity Identified by Using a High-Throughput Neutralization Assay together with an Analytical Selection Algorithm", Journal of Virology, vol. 83, pp. 7337-7348, 2009. http://dx.doi.org/10.1128/JVI.00110-09
- P. Kwong, and J. Mascola, "Human Antibodies that Neutralize HIV-1: Identification, Structures, and B Cell Ontogenies", Immunity, vol. 37, pp. 412-425, 2012. http://dx.doi.org/10.1016/j.immuni.2012.08.012
- M.A. Fareeha, "Development of IgG Mediated Antibody Dependent Cell-mediated Cytotoxicity (ADCC) in the Serum and Genital Mucosa of HIV Seroconverters", Journal of AIDS & Clinical Research, vol. 06, 2015. http://dx.doi.org/10.4172/2155-6113.1000479
- B. Su, and C. Moog, "Which Antibody Functions are Important for an HIV Vaccine?", Frontiers in Immunology, vol. 5, 2014. http://dx.doi.org/10.3389/fimmu.2014.00289
- K.L. Williams, V. Cortez, A.S. Dingens, J.S. Gach, S. Rainwater, J.F. Weis, X. Chen, P. Spearman, D.N. Forthal, and J. Overbaugh, "HIV-specific CD4-induced Antibodies Mediate Broad and Potent Antibody-dependent Cellular Cytotoxicity Activity and are Commonly Detected in Plasma from HIV-infected Humans", EBioMedicine, vol. 2, pp. 1464-1477, 2015. http://dx.doi.org/10.1016/j.ebiom.2015.09.001
- D.N. Forthal, G. Landucci, B. Chohan, B.A. Richardson, R.S. McClelland, W. Jaoko, C. Blish, and J. Overbaugh, "Antibody-Dependent Cell-Mediated Virus Inhibition Antibody Activity Does Not Correlate With Risk of HIV-1 Superinfection", JAIDS Journal of Acquired Immune Deficiency Syndromes, vol. 63, pp. 31-33, 2013. http://dx.doi.org/10.1097/QAI.0b013e3182874d41
- J.S. Gach, C.J. Achenbach, V. Chromikova, B. Berzins, N. Lambert, G. Landucci, D.N. Forthal, C. Katlama, B.H. Jung, and R.L. Murphy, "HIV-1 Specific Antibody Titers and Neutralization among Chronically Infected Patients on Long-Term Suppressive Antiretroviral Therapy (ART): A Cross-Sectional Study", PLoS ONE, vol. 9, pp. e85371, 2014. http://dx.doi.org/10.1371/journal.pone.0085371
- M. Sips, M. Krykbaeva, T. Diefenbach, M. Ghebremichael, B. Bowman, A. Dugast, A. Boesch, H. Streeck, D. Kwon, M. Ackerman, T. Suscovich, P. Brouckaert, T. Schacker, and G. Alter, "Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies", Mucosal Immunology, vol. 9, pp. 1584-1595, 2016. http://dx.doi.org/10.1038/mi.2016.12
- J.R. Mascola, and D.C. Montefiori, "The Role of Antibodies in HIV Vaccines", Annual Review of Immunology, vol. 28, pp. 413-444, 2010. http://dx.doi.org/10.1146/annurev-immunol-030409-101256
- G.J. Nabel, P.D. Kwong, and J.R. Mascola, "Progress in the rational design of an AIDS vaccine", Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 366, pp. 2759-2765, 2011. http://dx.doi.org/10.1098/rstb.2011.0096
- P.D. Kwong, J.R. Mascola, and G.J. Nabel, "Rational Design of Vaccines to Elicit Broadly Neutralizing Antibodies to HIV-1", Cold Spring Harbor Perspectives in Medicine, vol. 1, pp. a007278-a007278, 2011. http://dx.doi.org/10.1101/cshperspect.a007278
- A.C. Jung, and D.S. Paauw, "Diagnosing HIV-Related disease", Journal of General Internal Medicine, vol. 13, pp. 131-136, 1998. http://dx.doi.org/10.1046/j.1525-1497.1998.00031.x
- S. Tokman, and L. Huang, "Evaluation of Respiratory Disease", Clinics in Chest Medicine, vol. 34, pp. 191-204, 2013. http://dx.doi.org/10.1016/j.ccm.2013.02.005
- E.N. Janoff, R.F. Breiman, C.L. Daley, and P.C. Hopewell, "Pneumococcal Disease during HIV Infection", Annals of Internal Medicine, vol. 117, pp. 314-324, 1992. http://dx.doi.org/10.7326/0003-4819-117-4-314
- R.E. Hirschtick, J. Glassroth, M.C. Jordan, T.C. Wilcosky, J.M. Wallace, P.A. Kvale, N. Markowitz, M.J. Rosen, B.T. Mangura, and P.C. Hopewell, "Bacterial Pneumonia in Persons Infected with the Human Immunodeficiency Virus", New England Journal of Medicine, vol. 333, pp. 845-851, 1995. http://dx.doi.org/10.1056/NEJM199509283331305
- A.A. Lackner, M.M. Lederman, and B. Rodriguez, "HIV Pathogenesis: The Host", Cold Spring Harbor Perspectives in Medicine, vol. 2, pp. a007005-a007005, 2012. http://dx.doi.org/10.1101/cshperspect.a007005
- S.E. Langford, J. Ananworanich, and D.A. Cooper, "Predictors of disease progression in HIV infection: a review", AIDS Research and Therapy, vol. 4, pp. 11, 2007. http://dx.doi.org/10.1186/1742-6405-4-11
- A. Liovat, M. Rey-Cuillé, C. Lécuroux, B. Jacquelin, I. Girault, G. Petitjean, Y. Zitoun, A. Venet, F. Barré-Sinoussi, P. Lebon, L. Meyer, M. Sinet, and M. Müller-Trutwin, "Acute Plasma Biomarkers of T Cell Activation Set-Point Levels and of Disease Progression in HIV-1 Infection", PLoS ONE, vol. 7, pp. e46143, 2012. http://dx.doi.org/10.1371/journal.pone.0046143
-
M. Paiardini, and M. Müller‐Trutwin, "
HIV ‐associated chronic immune activation", Immunological Reviews, vol. 254, pp. 78-101, 2013. http://dx.doi.org/10.1111/imr.12079 - J.M. Brenchley, N.J. Karandikar, M.R. Betts, D.R. Ambrozak, B.J. Hill, L.E. Crotty, J.P. Casazza, J. Kuruppu, S.A. Migueles, M. Connors, M. Roederer, D.C. Douek, and R.A. Koup, "Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells", Blood, vol. 101, pp. 2711-2720, 2003. http://dx.doi.org/10.1182/blood-2002-07-2103
- C.L. Day, D.E. Kaufmann, P. Kiepiela, J.A. Brown, E.S. Moodley, S. Reddy, E.W. Mackey, J.D. Miller, A.J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E.J. Wherry, H.M. Coovadia, P.J.R. Goulder, P. Klenerman, R. Ahmed, G.J. Freeman, and B.D. Walker, "PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression", Nature, vol. 443, pp. 350-354, 2006. http://dx.doi.org/10.1038/nature05115
- N. Onlamoon, S. Tabprasit, S. Suwanagool, S. Louisirirotchanakul, A.A. Ansari, and K. Pattanapanyasat, "Studies on the potential use of CD38 expression as a marker for the efficacy of anti-retroviral therapy in HIV-1-infected patients in Thailand", Virology, vol. 341, pp. 238-247, 2005. http://dx.doi.org/10.1016/j.virol.2005.07.018
- N. Sachsenberg, A.S. Perelson, S. Yerly, G.A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin, "Turnover of CD4+ and CD8+ T Lymphocytes in HIV-1 Infection as Measured by Ki-67 Antigen", The Journal of Experimental Medicine, vol. 187, pp. 1295-1303, 1998. http://dx.doi.org/10.1084/jem.187.8.1295
- D.C. Douek, M.R. Betts, B.J. Hill, S.J. Little, R. Lempicki, J.A. Metcalf, J. Casazza, C. Yoder, J.W. Adelsberger, R.A. Stevens, M.W. Baseler, P. Keiser, D.D. Richman, R.T. Davey, and R.A. Koup, "Evidence for Increased T Cell Turnover and Decreased Thymic Output in HIV Infection", The Journal of Immunology, vol. 167, pp. 6663-6668, 2001. http://dx.doi.org/10.4049/jimmunol.167.11.6663
- J.A. Kovacs, R.A. Lempicki, I.A. Sidorov, J.W. Adelsberger, B. Herpin, J.A. Metcalf, I. Sereti, M.A. Polis, R.T. Davey, J. Tavel, J. Falloon, R. Stevens, L. Lambert, R. Dewar, D.J. Schwartzentruber, M.R. Anver, M.W. Baseler, H. Masur, D.S. Dimitrov, and H.C. Lane, "Identification of Dynamically Distinct Subpopulations of T Lymphocytes That Are Differentially Affected by HIV", The Journal of Experimental Medicine, vol. 194, pp. 1731-1741, 2001. http://dx.doi.org/10.1084/jem.194.12.1731
- S. Sieg, B. Rodriguez, R. Asaad, W. Jiang, D. Bazdar, and M. Lederman, "Peripheral S‐Phase T Cells in HIV Disease Have a Central Memory Phenotype and Rarely Have Evidence of Recent T Cell Receptor Engagement", The Journal of Infectious Diseases, vol. 192, pp. 62-70, 2005. http://dx.doi.org/10.1086/430620
- L.J. Picker, S.I. Hagen, R. Lum, E.F. Reed-Inderbitzin, L.M. Daly, A.W. Sylwester, J.M. Walker, D.C. Siess, M. Piatak, C. Wang, D.B. Allison, V.C. Maino, J.D. Lifson, T. Kodama, and M.K. Axthelm, "Insufficient Production and Tissue Delivery of CD4+Memory T Cells in Rapidly Progressive Simian Immunodeficiency Virus Infection", The Journal of Experimental Medicine, vol. 200, pp. 1299-1314, 2004. http://dx.doi.org/10.1084/jem.20041049
- J.A. Zack, S.J. Arrigo, S.R. Weitsman, A.S. Go, A. Haislip, and I.S. Chen, "HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure", Cell, vol. 61, pp. 213-222, 1990. http://dx.doi.org/10.1016/0092-8674(90)90802-l
- O. Ramilo, K.D. Bell, J.W. Uhr, and E.S. Vitetta, "Role of CD25+ and CD25-T cells in acute HIV infection in vitro.", Journal of immunology (Baltimore, Md. : 1950), 1993. http://www.ncbi.nlm.nih.gov/pubmed/8496611
- S.F. Sieg, D.A. Bazdar, and M.M. Lederman, "S-phase entry leads to cell death in circulating T cells from HIV-infected persons", Journal of Leukocyte Biology, vol. 83, pp. 1382-1387, 2008. http://dx.doi.org/10.1189/jlb.0907643
- A. Beignon, "Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor- viral RNA interactions", Journal of Clinical Investigation, vol. 115, pp. 3265-3275, 2005. http://dx.doi.org/10.1172/JCI26032
- A. Meier, and M. Altfeld, "Toll-like receptor signaling in HIV-1 infection: a potential target for therapy?", Expert Review of Anti-infective Therapy, vol. 5, pp. 323-326, 2007. http://dx.doi.org/10.1586/14787210.5.3.323
- N. Manel, B. Hogstad, Y. Wang, D.E. Levy, D. Unutmaz, and D.R. Littman, "A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells", Nature, vol. 467, pp. 214-217, 2010. http://dx.doi.org/10.1038/nature09337
- N. Yan, A.D. Regalado-Magdos, B. Stiggelbout, M.A. Lee-Kirsch, and J. Lieberman, "The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1", Nature Immunology, vol. 11, pp. 1005-1013, 2010. http://dx.doi.org/10.1038/ni.1941
- F.M. Shebl, K. Yu, O. Landgren, J.J. Goedert, and C.S. Rabkin, "Increased Levels of Circulating Cytokines with HIV-Related Immunosuppression", AIDS Research and Human Retroviruses, vol. 28, pp. 809-815, 2012. http://dx.doi.org/10.1089/AID.2011.0144
- P.W. Hunt, J.N. Martin, E. Sinclair, L. Epling, J. Teague, M.A. Jacobson, R.P. Tracy, L. Corey, and S.G. Deeks, "Valganciclovir Reduces T Cell Activation in HIV-Infected Individuals With Incomplete CD4+ T Cell Recovery on Antiretroviral Therapy", The Journal of Infectious Diseases, vol. 203, pp. 1474-1483, 2011. http://dx.doi.org/10.1093/infdis/jir060
- A. Okoye, M. Meier-Schellersheim, J.M. Brenchley, S.I. Hagen, J.M. Walker, M. Rohankhedkar, R. Lum, J.B. Edgar, S.L. Planer, A. Legasse, A.W. Sylwester, M. Piatak, J.D. Lifson, V.C. Maino, D.L. Sodora, D.C. Douek, M.K. Axthelm, Z. Grossman, and L.J. Picker, "Progressive CD4+ central–memory T cell decline results in CD4+ effector–memory insufficiency and overt disease in chronic SIV infection", The Journal of Experimental Medicine, vol. 204, pp. 2171-2185, 2007. http://dx.doi.org/10.1084/jem.20070567
- J.M. Brenchley, D.A. Price, T.W. Schacker, T.E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B.R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J.N. Martin, F.M. Hecht, L.J. Picker, M.M. Lederman, S.G. Deeks, and D.C. Douek, "Microbial translocation is a cause of systemic immune activation in chronic HIV infection", Nature Medicine, vol. 12, pp. 1365-1371, 2006. http://dx.doi.org/10.1038/nm1511
- N. Funderburg, A.A. Luciano, W. Jiang, B. Rodriguez, S.F. Sieg, and M.M. Lederman, "Toll-Like Receptor Ligands Induce Human T Cell Activation and Death, a Model for HIV Pathogenesis", PLoS ONE, vol. 3, pp. e1915, 2008. http://dx.doi.org/10.1371/journal.pone.0001915
- D.L. Sodora, J.S. Allan, C. Apetrei, J.M. Brenchley, D.C. Douek, J.G. Else, J.D. Estes, B.H. Hahn, V.M. Hirsch, A. Kaur, F. Kirchhoff, M. Muller-Trutwin, I. Pandrea, J.E. Schmitz, and G. Silvestri, "Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts", Nature Medicine, vol. 15, pp. 861-865, 2009. http://dx.doi.org/10.1038/nm.2013
- J.D. Estes, S.N. Gordon, M. Zeng, A.M. Chahroudi, R.M. Dunham, S.I. Staprans, C.S. Reilly, G. Silvestri, and A.T. Haase, "Early Resolution of Acute Immune Activation and Induction of PD-1 in SIV-Infected Sooty Mangabeys Distinguishes Nonpathogenic from Pathogenic Infection in Rhesus Macaques", The Journal of Immunology, vol. 180, pp. 6798-6807, 2008. http://dx.doi.org/10.4049/jimmunol.180.10.6798
- M. Meythaler, A. Martinot, Z. Wang, S. Pryputniewicz, M. Kasheta, B. Ling, P.A. Marx, S. O'Neil, and A. Kaur, "Differential CD4 + T-Lymphocyte Apoptosis and Bystander T-Cell Activation in Rhesus Macaques and Sooty Mangabeys during Acute Simian Immunodeficiency Virus Infection", Journal of Virology, vol. 83, pp. 572-583, 2009. http://dx.doi.org/10.1128/JVI.01715-08
- S. Lederer, D. Favre, K. Walters, S. Proll, B. Kanwar, Z. Kasakow, C.R. Baskin, R. Palermo, J.M. McCune, and M.G. Katze, "Transcriptional Profiling in Pathogenic and Non-Pathogenic SIV Infections Reveals Significant Distinctions in Kinetics and Tissue Compartmentalization", PLoS Pathogens, vol. 5, pp. e1000296, 2009. http://dx.doi.org/10.1371/journal.ppat.1000296
- T. Bruel, S. Dupuy, T. Démoulins, C. Rogez-Kreuz, J. Dutrieux, A. Corneau, A. Cosma, R. Cheynier, N. Dereuddre-Bosquet, R. Le Grand, and B. Vaslin, "Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques", PLoS Pathogens, vol. 10, pp. e1003915, 2014. http://dx.doi.org/10.1371/journal.ppat.1003915
- G.A.D. Hardy, S. Sieg, B. Rodriguez, D. Anthony, R. Asaad, W. Jiang, J. Mudd, T. Schacker, N.T. Funderburg, H.A. Pilch-Cooper, R. Debernardo, R.L. Rabin, M.M. Lederman, and C.V. Harding, "Interferon-α Is the Primary Plasma Type-I IFN in HIV-1 Infection and Correlates with Immune Activation and Disease Markers", PLoS ONE, vol. 8, pp. e56527, 2013. http://dx.doi.org/10.1371/journal.pone.0056527
- J. Alimonti, S. Koesters, J. Kimani, L. Matu, C. Wachihi, F. Plummer, and K. Fowke, "CD4+T Cell Responses in HIV‐Exposed Seronegative Women Are Qualitatively Distinct from Those in HIV‐Infected Women", The Journal of Infectious Diseases, vol. 191, pp. 20-24, 2005. http://dx.doi.org/10.1086/425998
- J.B. Alimonti, J. Kimani, L. Matu, C. Wachihi, R. Kaul, F.A. Plummer, and K.R. Fowke, "Characterization of CD8+ T‐cell responses in HIV‐1‐exposed seronegative commercial sex workers from Nairobi, Kenya", Immunology & Cell Biology, vol. 84, pp. 482-485, 2006. http://dx.doi.org/10.1111/j.1440-1711.2006.01455.x
- R. Hardie, M. Luo, B. Bruneau, E. Knight, N.J. Nagelkerke, J. Kimani, C. Wachihi, E.N. Ngugi, and F.A. Plummer, "Human leukocyte antigen-DQ alleles and haplotypes and their associations with resistance and susceptibility to HIV-1 infection", AIDS, vol. 22, pp. 807-816, 2008. http://dx.doi.org/10.1097/QAD.0b013e3282f51b71
- P.A. Lacap, J.D. Huntington, M. Luo, N.J. Nagelkerke, T. Bielawny, J. Kimani, C. Wachihi, E.N. Ngugi, and F.A. Plummer, "Associations of human leukocyte antigen DRB with resistance or susceptibility to HIV-1 infection in the Pumwani Sex Worker Cohort", AIDS, vol. 22, pp. 1029-1038, 2008. http://dx.doi.org/10.1097/QAD.0b013e3282ffb3db
- K. MacDonald, K. Fowke, J. Kimani, V. Dunand, N. Nagelkerke, T. Ball, J. Oyugi, E. Njagi, L. Gaur, R. Brunham, J. Wade, M. Luscher, P. Krausa, S. Rowland‐Jones, E. Ngugi, J. Bwayo, and F. Plummer, "Influence of HLA Supertypes on Susceptibility and Resistance to Human Immunodeficiency Virus Type 1 Infection", The Journal of Infectious Diseases, vol. 181, pp. 1581-1589, 2000. http://dx.doi.org/10.1086/315472
- T.B. Ball, H. Ji, J. Kimani, P. McLaren, C. Marlin, A.V. Hill, and F.A. Plummer, "Polymorphisms in IRF-1 associated with resistance to HIV-1 infection in highly exposed uninfected Kenyan sex workers", AIDS, vol. 21, pp. 1091-1101, 2007. http://dx.doi.org/10.1097/QAD.0b013e3280ef6ae1
- P. McLaren, T. Ball, C. Wachihi, W. Jaoko, D. Kelvin, A. Danesh, J. Kimani, F. Plummer, and K. Fowke, "HIV‐Exposed Seronegative Commercial Sex Workers Show a Quiescent Phenotype in the CD4+T Cell Compartment and Reduced Expression of HIV‐Dependent Host Factors", The Journal of Infectious Diseases, vol. 202, pp. S339-S344, 2010. http://dx.doi.org/10.1086/655968
- F.A. Koning, T.J.K. van der Vorst, and H. Schuitemaker, "Low Levels of Human Immunodeficiency Virus Type 1 DNA in High-Risk Seronegative Men", Journal of Virology, vol. 79, pp. 6551-6553, 2005. http://dx.doi.org/10.1128/JVI.79.10.6551-6553.2005
- W. Jennes, D. Evertse, M. Borget, B. Vuylsteke, C. Maurice, J.N. Nkengasong, and L. Kestens, "Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers", Clinical and Experimental Immunology, vol. 143, pp. 435-444, 2006. http://dx.doi.org/10.1111/j.1365-2249.2006.03017.x
- S. Broder, "The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic", Antiviral Research, vol. 85, pp. 1-18, 2010. http://dx.doi.org/10.1016/j.antiviral.2009.10.002
- D.A. Kulpa, and N. Chomont, "HIV persistence in the setting of antiretroviral therapy: when, where and how does HIV hide?", Journal of virus eradication, 2015. http://www.ncbi.nlm.nih.gov/pubmed/26448966
- M.L. Fernandez Guerrero, P. Rivas, M. Molina, R. Garcia, and M. De Gorgolas, "Long-Term Follow-Up of Asymptomatic HIV-Infected Patients Who Discontinued Antiretroviral Therapy", Clinical Infectious Diseases, vol. 41, pp. 390-394, 2005. http://dx.doi.org/10.1086/431487
- N. Ford, A. Calmy, and S. Hurst, "When to start antiretroviral therapy in resource-limited settings: a human rights analysis", BMC International Health and Human Rights, vol. 10, 2010. http://dx.doi.org/10.1186/1472-698x-10-6
- S.G. Deeks, "Determinants of Virological Response to Antiretroviral Therapy: Implications for Long-Term Strategies", Clinical Infectious Diseases, vol. 30, pp. S177-S184, 2000. http://dx.doi.org/10.1086/313855
- P.K. Lee, T.L. Kieffer, R.F. Siliciano, and R.E. Nettles, "HIV-1 viral load blips are of limited clinical significance", Journal of Antimicrobial Chemotherapy, vol. 57, pp. 803-805, 2006. http://dx.doi.org/10.1093/jac/dkl092
- S. Bertozzi, N. Padian, J. Wegbreit, L. DeMaria, B. Feldman, H. Gayle, J. Gold, R. Grant, and M. Isbell, "HIV/AIDS Prevention and Treatment.", Disease Control Priorities in Developing Countries, 2nd Edition, pp. 331-369., 2006.
- J.E. Volk, J.L. Marcus, T. Phengrasamy, D. Blechinger, D.P. Nguyen, S. Follansbee, and C.B. Hare, "No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting: Figure 1.", Clinical Infectious Diseases, vol. 61, pp. 1601-1603, 2015. http://dx.doi.org/10.1093/cid/civ778
- C.A. Sabin, and A.N. Phillips, "Should HIV therapy be started at a CD4 cell count above 350 cells/μl in asymptomatic HIV-1-infected patients?", Current Opinion in Infectious Diseases, vol. 22, pp. 191-197, 2009. http://dx.doi.org/10.1097/QCO.0b013e328326cd34
- V. Jain, and S.G. Deeks, "When to Start Antiretroviral Therapy", Current HIV/AIDS Reports, vol. 7, pp. 60-68, 2010. http://dx.doi.org/10.1007/s11904-010-0044-6
- R.A. Franco, and M.S. Saag, "When to start antiretroviral therapy: as soon as possible", BMC Medicine, vol. 11, 2013. http://dx.doi.org/10.1186/1741-7015-11-147
- J.D. Lundgren, A.G. Babiker, F.M. Gordin, �.H. Borges, and J.D. Neaton, "When to start antiretroviral therapy: the need for an evidence base during early HIV infection", BMC Medicine, vol. 11, 2013. http://dx.doi.org/10.1186/1741-7015-11-148
- . , "A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa", New England Journal of Medicine, vol. 373, pp. 808-822, 2015. http://dx.doi.org/10.1056/NEJMoa1507198
-
N. Geffen, P. Aagaard, G. Corbelli, M. Meulbroek, D. Peavy, C. Rappoport, S. Schwarze, S. Collins, and . , "Community perspective on the
INSIGHT S trategicT iming ofAntiRetroviral T reatment (START ) trial", HIV Medicine, vol. 16, pp. 10-13, 2015. http://dx.doi.org/10.1111/hiv.12228 - S.S. Abdool Karim, "Overcoming Impediments to Global Implementation of Early Antiretroviral Therapy", New England Journal of Medicine, vol. 373, pp. 875-876, 2015. http://dx.doi.org/10.1056/Nejme1508527
- M. Lahuerta, F. Ue, S. Hoffman, B. Elul, S.G. Kulkarni, Y. Wu, H. Nuwagaba-Biribonwoha, R.H. Remien, W. El Sadr, and D. Nash, "The Problem of Late ART Initiation in Sub-Saharan Africa: A Transient Aspect of Scale-up or a Long-term Phenomenon?", Journal of Health Care for the Poor and Underserved, vol. 24, pp. 359-383, 2013. http://dx.doi.org/10.1353/hpu.2013.0014
- M. May, M. Gompels, V. Delpech, K. Porter, F. Post, M. Johnson, D. Dunn, A. Palfreeman, R. Gilson, B. Gazzard, T. Hill, J. Walsh, M. Fisher, C. Orkin, J. Ainsworth, L. Bansi, A. Phillips, C. Leen, M. Nelson, J. Anderson, and C. Sabin, "Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study", BMJ, vol. 343, pp. d6016-d6016, 2011. http://dx.doi.org/10.1136/Bmj.D6016
- J.B. Nachega, J. Parienti, O.A. Uthman, R. Gross, D.W. Dowdy, P.E. Sax, J.E. Gallant, M.J. Mugavero, E.J. Mills, and T.P. Giordano, "Lower Pill Burden and Once-Daily Antiretroviral Treatment Regimens for HIV Infection: A Meta-Analysis of Randomized Controlled Trials", Clinical Infectious Diseases, vol. 58, pp. 1297-1307, 2014. http://dx.doi.org/10.1093/cid/ciu046
- P.S. Pennings, "HIV drug resistance: problems and perspectives", Infectious Disease Reports, vol. 5, pp. e5, 2013. http://dx.doi.org/10.4081/idr.2013.s1.e5
- O. Keiser, C. Orrell, M. Egger, R. Wood, M.W.G. Brinkhof, H. Furrer, G. van Cutsem, B. Ledergerber, A. Boulle, and . , "Public-Health and Individual Approaches to Antiretroviral Therapy: Township South Africa and Switzerland Compared", PLoS Medicine, vol. 5, pp. e148, 2008. http://dx.doi.org/10.1371/journal.pmed.0050148
- C.V. Fletcher, K. Staskus, S.W. Wietgrefe, M. Rothenberger, C. Reilly, J.G. Chipman, G.J. Beilman, A. Khoruts, A. Thorkelson, T.E. Schmidt, J. Anderson, K. Perkey, M. Stevenson, A.S. Perelson, D.C. Douek, A.T. Haase, and T.W. Schacker, "Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues", Proceedings of the National Academy of Sciences, vol. 111, pp. 2307-2312, 2014. http://dx.doi.org/10.1073/pnas.1318249111
- L.R. McKinnon, M. Kimani, C. Wachihi, N.J. Nagelkerke, F.K. Muriuki, A. Kariri, R.T. Lester, L. Gelmon, T.B. Ball, F.A. Plummer, R. Kaul, and J. Kimani, "Effect of Baseline HIV Disease Parameters on CD4+ T Cell Recovery After Antiretroviral Therapy Initiation in Kenyan Women", PLoS ONE, vol. 5, pp. e11434, 2010. http://dx.doi.org/10.1371/journal.pone.0011434
- S. Serrano-Villar, T. Sainz, S.A. Lee, P.W. Hunt, E. Sinclair, B.L. Shacklett, A.L. Ferre, T.L. Hayes, M. Somsouk, P.Y. Hsue, M.L. Van Natta, C.L. Meinert, M.M. Lederman, H. Hatano, V. Jain, Y. Huang, F.M. Hecht, J.N. Martin, J.M. McCune, S. Moreno, and S.G. Deeks, "HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality", PLoS Pathogens, vol. 10, pp. e1004078, 2014. http://dx.doi.org/10.1371/journal.ppat.1004078
- S. Serrano-Villar, M.J. Pérez-Elías, F. Dronda, J.L. Casado, A. Moreno, A. Royuela, J.A. Pérez-Molina, T. Sainz, E. Navas, J.M. Hermida, C. Quereda, and S. Moreno, "Increased Risk of Serious Non-AIDS-Related Events in HIV-Infected Subjects on Antiretroviral Therapy Associated with a Low CD4/CD8 Ratio", PLoS ONE, vol. 9, pp. e85798, 2014. http://dx.doi.org/10.1371/journal.pone.0085798
- A. Boulle, M. Schomaker, M.T. May, R.S. Hogg, B.E. Shepherd, S. Monge, O. Keiser, F.C. Lampe, J. Giddy, J. Ndirangu, D. Garone, M. Fox, S.M. Ingle, P. Reiss, F. Dabis, D. Costagliola, A. Castagna, K. Ehren, C. Campbell, M.J. Gill, M. Saag, A.C. Justice, J. Guest, H.M. Crane, M. Egger, and J.A.C. Sterne, "Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies", PLoS Medicine, vol. 11, pp. e1001718, 2014. http://dx.doi.org/10.1371/journal.pmed.1001718
- M.T. May, M. Gompels, V. Delpech, K. Porter, C. Orkin, S. Kegg, P. Hay, M. Johnson, A. Palfreeman, R. Gilson, D. Chadwick, F. Martin, T. Hill, J. Walsh, F. Post, M. Fisher, J. Ainsworth, S. Jose, C. Leen, M. Nelson, J. Anderson, and C. Sabin, "Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy", AIDS, vol. 28, pp. 1193-1202, 2014. http://dx.doi.org/10.1097/QAD.0000000000000243
- D.I.S. Rosenbloom, A.L. Hill, S.A. Rabi, R.F. Siliciano, and M.A. Nowak, "Antiretroviral dynamics determines HIV evolution and predicts therapy outcome", Nature Medicine, vol. 18, pp. 1378-1385, 2012. http://dx.doi.org/10.1038/nm.2892
- E.L. Asahchop, M.A. Wainberg, R.D. Sloan, and C.L. Tremblay, "Antiviral Drug Resistance and the Need for Development of New HIV-1 Reverse Transcriptase Inhibitors", Antimicrobial Agents and Chemotherapy, vol. 56, pp. 5000-5008, 2012. http://dx.doi.org/10.1128/AAC.00591-12
- D. Frentz, C.A.B. Boucher, and D.A.M.C. van de Vijver, "Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world.", AIDS reviews, 2012. http://www.ncbi.nlm.nih.gov/pubmed/22297501
- M.A. Wainberg, and B.G. Brenner, "Role of HIV Subtype Diversity in the Development of Resistance to Antiviral Drugs", Viruses, vol. 2, pp. 2493-2508, 2010. http://dx.doi.org/10.3390/v2112493
- B.G. Brenner, M. Oliveira, F. Doualla-Bell, D.D. Moisi, M. Ntemgwa, F. Frankel, M. Essex, and M.A. Wainberg, "HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture", AIDS, vol. 20, pp. F9-F13, 2006. http://dx.doi.org/10.1097/01.aids.0000232228.88511.0b
- R.K. Gupta, I.L. Chrystie, S. O'Shea, J.E. Mullen, R. Kulasegaram, and C.Y. Tong, "K65R and Y181C are less prevalent in HAART-experienced HIV-1 subtype A patients", AIDS, vol. 19, pp. 1916-1919, 2005. http://dx.doi.org/10.1097/01.aids.0000189860.36688.e5
- M.A. Wainberg, and B.G. Brenner, "The Impact of HIV Genetic Polymorphisms and Subtype Differences on the Occurrence of Resistance to Antiretroviral Drugs", Molecular Biology International, vol. 2012, pp. 1-10, 2012. http://dx.doi.org/10.1155/2012/256982
- M. Marsden, and J. Zack, "Double Trouble: HIV Latency and CTL Escape", Cell Host & Microbe, vol. 17, pp. 141-142, 2015. http://dx.doi.org/10.1016/j.chom.2015.01.008
- K. Deng, M. Pertea, A. Rongvaux, L. Wang, C.M. Durand, G. Ghiaur, J. Lai, H.L. McHugh, H. Hao, H. Zhang, J.B. Margolick, C. Gurer, A.J. Murphy, D.M. Valenzuela, G.D. Yancopoulos, S.G. Deeks, T. Strowig, P. Kumar, J.D. Siliciano, S.L. Salzberg, R.A. Flavell, L. Shan, and R.F. Siliciano, "Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations", Nature, vol. 517, pp. 381-385, 2015. http://dx.doi.org/10.1038/nature14053
- L. Shan, and R.F. Siliciano, "From reactivation of latent HIV‐1 to elimination of the latent reservoir: The presence of multiple barriers to viral eradication", BioEssays, vol. 35, pp. 544-552, 2013. http://dx.doi.org/10.1002/bies.201200170
- M.D. Marsden, and J.A. Zack, "HIV/AIDS eradication", Bioorganic & Medicinal Chemistry Letters, vol. 23, pp. 4003-4010, 2013. http://dx.doi.org/10.1016/j.bmcl.2013.05.032
- L. Shan, K. Deng, N. Shroff, C. Durand, S. Rabi, H. Yang, H. Zhang, J. Margolick, J. Blankson, and R. Siliciano, "Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation", Immunity, vol. 36, pp. 491-501, 2012. http://dx.doi.org/10.1016/j.immuni.2012.01.014
- C. Liu, X. Ma, B. Liu, C. Chen, and H. Zhang, "HIV-1 functional cure: will the dream come true?", BMC Medicine, vol. 13, 2015. http://dx.doi.org/10.1186/s12916-015-0517-y
- C.B. Wilen, J.C. Tilton, and R.W. Doms, "HIV: Cell Binding and Entry", Cold Spring Harbor Perspectives in Medicine, vol. 2, pp. a006866-a006866, 2012. http://dx.doi.org/10.1101/cshperspect.a006866
- M.R. Tardif, and M.J. Tremblay, "Presence of Host ICAM-1 in Human Immunodeficiency Virus Type 1 Virions Increases Productive Infection of CD4 + T Lymphocytes by Favoring Cytosolic Delivery of Viral Material", Journal of Virology, vol. 77, pp. 12299-12309, 2003. http://dx.doi.org/10.1128/jvi.77.22.12299-12309.2003
- J.D. Lifson, and E.G. Engleman, "Role of CD4 in Normal Immunity and HIV Infection", Immunological Reviews, vol. 109, pp. 93-117, 1989. http://dx.doi.org/10.1111/j.1600-065x.1989.tb00021.x
-
K.J. Doores, "The
HIV glycan shield as a target for broadly neutralizing antibodies", The FEBS Journal, vol. 282, pp. 4679-4691, 2015. http://dx.doi.org/10.1111/febs.13530 - E.Y. Dotsey, A. Gorlani, S. Ingale, C.J. Achenbach, D.N. Forthal, P.L. Felgner, and J.S. Gach, "A High Throughput Protein Microarray Approach to Classify HIV Monoclonal Antibodies and Variant Antigens", PLOS ONE, vol. 10, pp. e0125581, 2015. http://dx.doi.org/10.1371/journal.pone.0125581
- D. Vigerust, "Protein glycosylation in infectious disease pathobiology and treatment", Open Life Sciences, vol. 6, pp. 802-816, 2011. http://dx.doi.org/10.2478/s11535-011-0050-8
- J.S. Gach, H. Quendler, T. Tong, K.M. Narayan, S.X. Du, R.G. Whalen, J.M. Binley, D.N. Forthal, P. Poignard, and M.B. Zwick, "A Human Antibody to the CD4 Binding Site of gp120 Capable of Highly Potent but Sporadic Cross Clade Neutralization of Primary HIV-1", PLoS ONE, vol. 8, pp. e72054, 2013. http://dx.doi.org/10.1371/journal.pone.0072054
- N.G. Hoffman, F. Seillier-Moiseiwitsch, J. Ahn, J.M. Walker, and R. Swanstrom, "Variability in the Human Immunodeficiency Virus Type 1 gp120 Env Protein Linked to Phenotype-Associated Changes in the V3 Loop", Journal of Virology, vol. 76, pp. 3852-3864, 2002. http://dx.doi.org/10.1128/Jvi.76.8.3852-3864.2002
- R. Wyatt, P.D. Kwong, E. Desjardins, R.W. Sweet, J. Robinson, W.A. Hendrickson, and J.G. Sodroski, "The antigenic structure of the HIV gp120 envelope glycoprotein", Nature, vol. 393, pp. 705-711, 1998. http://dx.doi.org/10.1038/31514
- P.D. Kwong, R. Wyatt, J. Robinson, R.W. Sweet, J. Sodroski, and W.A. Hendrickson, "Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody", Nature, vol. 393, pp. 648-659, 1998. http://dx.doi.org/10.1038/31405
- J. Garcia-Perez, I. Staropoli, S. Azoulay, J. Heinrich, A. Cascajero, P. Colin, H. Lortat-Jacob, F. Arenzana-Seisdedos, J. Alcami, E. Kellenberger, and B. Lagane, "A single-residue change in the HIV-1 V3 loop associated with maraviroc resistance impairs CCR5 binding affinity while increasing replicative capacity", Retrovirology, vol. 12, 2015. http://dx.doi.org/10.1186/s12977-015-0177-1
- R.W. Doms, and J.P. Moore, "HIV-1 Membrane Fusion", The Journal of Cell Biology, vol. 151, pp. F9-F14, 2000. http://dx.doi.org/10.1083/jcb.151.2.f9
- M. Marmor, K. Hertzmark, S.M. Thomas, P.N. Halkitis, and M. Vogler, "Resistance to HIV Infection", Journal of Urban Health, vol. 83, pp. 5-17, 2006. http://dx.doi.org/10.1007/s11524-005-9003-8
- E.A. Berger, R.W. Doms, E. Fenyö, B.T.M. Korber, D.R. Littman, J.P. Moore, Q.J. Sattentau, H. Schuitemaker, J. Sodroski, and R.A. Weiss, "A new classification for HIV-1", Nature, vol. 391, pp. 240-240, 1998. http://dx.doi.org/10.1038/34571
- J. Grivel, R.J. Shattock, and L.B. Margolis, "Selective transmission of R5 HIV-1 variants: where is the gatekeeper?", Journal of Translational Medicine, vol. 9, 2011. http://dx.doi.org/10.1186/1479-5876-9-S1-S6
- M.J. Lehmann, N.M. Sherer, C.B. Marks, M. Pypaert, and W. Mothes, "Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells", The Journal of Cell Biology, vol. 170, pp. 317-325, 2005. http://dx.doi.org/10.1083/jcb.200503059
- C.B. Coyne, and J.M. Bergelson, "Virus-Induced Abl and Fyn Kinase Signals Permit Coxsackievirus Entry through Epithelial Tight Junctions", Cell, vol. 124, pp. 119-131, 2006. http://dx.doi.org/10.1016/j.cell.2005.10.035
- N.M. Sherer, J. Jin, and W. Mothes, "Directional Spread of Surface-Associated Retroviruses Regulated by Differential Virus-Cell Interactions", Journal of Virology, vol. 84, pp. 3248-3258, 2010. http://dx.doi.org/10.1128/JVI.02155-09
- W. Mothes, N.M. Sherer, J. Jin, and P. Zhong, "Virus Cell-to-Cell Transmission", Journal of Virology, vol. 84, pp. 8360-8368, 2010. http://dx.doi.org/10.1128/JVI.00443-10
- K. Miyauchi, Y. Kim, O. Latinovic, V. Morozov, and G.B. Melikyan, "HIV Enters Cells via Endocytosis and Dynamin-Dependent Fusion with Endosomes", Cell, vol. 137, pp. 433-444, 2009. http://dx.doi.org/10.1016/j.cell.2009.02.046
- J. S. Gach, D. P. Leaman, and M. B. Zwick, "Targeting HIV-1 gp41 in Close Proximity to the Membrane Using Antibody and Other Molecules", Current Topics in Medicinal Chemistry, vol. 11, pp. 2997-3021, 2011. http://dx.doi.org/10.2174/156802611798808505
- G.B. Melikyan, "Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm", Retrovirology, vol. 5, pp. 111, 2008. http://dx.doi.org/10.1186/1742-4690-5-111
- N. Martin, S. Welsch, C. Jolly, J.A.G. Briggs, D. Vaux, and Q.J. Sattentau, "Virological Synapse-Mediated Spread of Human Immunodeficiency Virus Type 1 between T Cells Is Sensitive to Entry Inhibition", Journal of Virology, vol. 84, pp. 3516-3527, 2010. http://dx.doi.org/10.1128/JVI.02651-09
- I.A. Abela, L. Berlinger, M. Schanz, L. Reynell, H.F. Günthard, P. Rusert, and A. Trkola, "Cell-Cell Transmission Enables HIV-1 to Evade Inhibition by Potent CD4bs Directed Antibodies", PLoS Pathogens, vol. 8, pp. e1002634, 2012. http://dx.doi.org/10.1371/journal.ppat.1002634
- C. Jolly, "Cell-to-cell transmission of retroviruses: Innate immunity and interferon-induced restriction factors", Virology, vol. 411, pp. 251-259, 2011. http://dx.doi.org/10.1016/j.virol.2010.12.031
- P. Chen, W. Hübner, M.A. Spinelli, and B.K. Chen, "Predominant Mode of Human Immunodeficiency Virus Transfer between T Cells Is Mediated by Sustained Env-Dependent Neutralization-Resistant Virological Synapses", Journal of Virology, vol. 81, pp. 12582-12595, 2007. http://dx.doi.org/10.1128/JVI.00381-07
- D. Mazurov, A. Ilinskaya, G. Heidecker, P. Lloyd, and D. Derse, "Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors", PLoS Pathogens, vol. 6, pp. e1000788, 2010. http://dx.doi.org/10.1371/journal.ppat.1000788
- R.L. Felts, K. Narayan, J.D. Estes, D. Shi, C.M. Trubey, J. Fu, L.M. Hartnell, G.T. Ruthel, D.K. Schneider, K. Nagashima, J.W. Bess, S. Bavari, B.C. Lowekamp, D. Bliss, J.D. Lifson, and S. Subramaniam, "3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells", Proceedings of the National Academy of Sciences, vol. 107, pp. 13336-13341, 2010. http://dx.doi.org/10.1073/pnas.1003040107
- T.B. Geijtenbeek, D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, C.G. Figdor, and Y. van Kooyk, "DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells", Cell, vol. 100, pp. 587-597, 2000. http://dx.doi.org/10.1016/s0092-8674(00)80694-7
- D. McDonald, L. Wu, S.M. Bohks, V.N. KewalRamani, D. Unutmaz, and T.J. Hope, "Recruitment of HIV and Its Receptors to Dendritic Cell-T Cell Junctions", Science, vol. 300, pp. 1295-1297, 2003. http://dx.doi.org/10.1126/science.1084238
- S.G. Turville, P.U. Cameron, A. Handley, G. Lin, S. Pöhlmann, R.W. Doms, and A.L. Cunningham, "Diversity of receptors binding HIV on dendritic cell subsets", Nature Immunology, vol. 3, pp. 975-983, 2002. http://dx.doi.org/10.1038/ni841
- W. Hübner, G.P. McNerney, P. Chen, B.M. Dale, R.E. Gordon, F.Y.S. Chuang, X. Li, D.M. Asmuth, T. Huser, and B.K. Chen, "Quantitative 3D Video Microscopy of HIV Transfer Across T Cell Virological Synapses", Science, vol. 323, pp. 1743-1747, 2009. http://dx.doi.org/10.1126/science.1167525
COPYRIGHT
© 2016

Recent Insights into the HIV/AIDS Pandemic by Becerra et al. is licensed under a Creative Commons Attribution 4.0 International License.