Research Reports:
Microbial Cell, Vol. 4, No. 6, pp. 191 - 199; doi: 10.15698/mic2017.06.577
The neuroprotective steroid progesterone promotes mitochondrial uncoupling, reduces cytosolic calcium and augments stress resistance in yeast cells
1 Institute of Molecular Biosciences, NAWI Graz, University of Graz, 8010 Graz, Austria.
2 BioTechMed Graz, Austria.
3 Equipe 11 labellisée par la Ligue contre le Cancer, Centre de Recherche des Cordeliers, Paris, France.
4 INSERM, U1138, Paris, France.
5 Université Paris Descartes, Sorbonne Paris Cité, Paris, France.
6 Cell Biology & Metabolomics Platforms, Gustave Roussy Comprehensive Cancer Center, Villejuif, France.
7 Pôle de Biologie, Hôpital Européen Georges Pompidou, AP‐HP, Paris, France.
8 Karolinska Institute, Department of Women’s and Children’s Health, Karolinska University Hospital, 17176 Stockholm, Sweden.
* These authors contributed equally.
Keywords: TBI; traumatic brain injury, cell protection, cell stress, cell death, neuroprotection, progesterone, mitochondrial uncoupling.
Received originally: 10/03/2017 Received in revised form: 21/05/2017
Accepted: 22/05/2017
Published: 31/05/2017
Correspondence:
Frank Madeo, Institute of Molecular Biosciences, University of Graz, Humboldtstrasse 50; 8010 Graz, Austria frank.madeo@uni-graz.at
Christoph Ruckenstuhl, Institute of Molecular Biosciences, University of Graz, Humboldtstrasse 50; 8010 Graz, Austria ru.ruckenstuhl@uni-graz.at
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Slaven Stekovic, Christoph Ruckenstuhl, Philipp Royer, Christof Winkler-Hermaden, Didac Carmona-Gutierrez, Kai-Uwe Fröhlich, Guido Kroemer, and Frank Madeo (2017). The neuroprotective steroid progesterone promotes mitochondrial uncoupling, reduces cytosolic calcium and augments stress resistance in yeast cells. Microbial Cell 4(6):191-199. doi: 10.15698/mic2017.06.577
Abstract
The steroid hormone progesterone is not only a crucial sex hormone, but also serves as a neurosteroid, thus playing an important role in brain function. Epidemiological data suggest that progesterone improves the recovery of patients after traumatic brain injury. Brain injuries are often connected to elevated calcium spikes, reactive oxygen species (ROS) and programmed cell death affecting neurons. Here, we establish a yeast model to study progesterone-mediated cytoprotection. External supply of progesterone protected yeast cells from apoptosis-inducing stress stimuli and resulted in elevated mitochondrial oxygen uptake accompanied by a drop in ROS generation and ATP levels during chronological aging. In addition, cellular Ca2+ concentrations were reduced upon progesterone treatment, and this effect occurred independently of known Ca2+ transporters and mitochondrial respiration. All effects were also independent of Dap1, the yeast orthologue of the progesterone receptor. Altogether, our observations provide new insights into the cytoprotective effects of progesterone.
INTRODUCTION
Progesterone is a sterol-derived hormone that is crucial for female reproductive capacity and plays major regulatory roles in the monthly menstrual cycle and upon conception as well as during pregnancy and embryogenesis. In addition, it also serves as a neurosteroid, thus playing an important role in brain function in both sexes [1]. For instance, progesterone inhibits the neuronal nicotinic acetylcholine receptor and stimulates the synthesis of myelin proteins [1]. Of note, progesterone has been linked to the gender-specific risk and outcome of brain injuries that is more favorable for females [2]. Interestingly, preclinical data strongly suggest that (high doses of) progesterone may positively affect recovery from traumatic brain injury (TBI) in model organisms [3][4][5][6][7], if administered before or shortly after TBI. Two clinical studies could confirm a neuroprotective effect of progesterone when administered shortly after TBI [8][9], while some more recent clinical data seem to disprove this hypothesis [10][11][12]. Therefore, it remains an open question if progesterone affects the recovery and survival after TBI in humans and to which extent it promotes cellular restauration.
–
In order to investigate the cytoprotective potential of progesterone, we took advantage of Saccharomyces cerevisiae, knowing that this organism has repeatedly been shown to be suitable for mechanistic studies of programmed cell death (PCD) [13][14][15][16][17][18][19]. Yeast is especially useful as a model to study neuroprotection at the cellular level [20][21][22][23][24][25][26][27]. Here, we describe the positive impact of progesterone on several parameters of cellular physiology. Importantly, our results also suggest a possible receptor-independent mechanism for these effects, since deletion of DAP1 – a heme-binding protein related to the mammalian membrane progesterone receptor – did not alter susceptibility towards progesterone treatment. Altogether, we reveal that progesterone exerts potent cytoprotective effects in yeast.
RESULTS
Progesterone increases stress tolerance
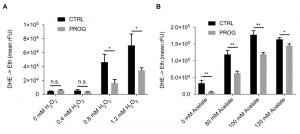
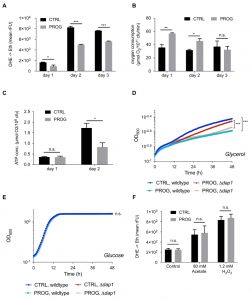
Traumatic brain injury is connected to elevated PCD and ROS accumulation in the brain tissue [28][29]. Therefore, we tested if progesterone would render yeast cells less susceptible towards different stressors that are connected to an increase in ROS production. Upon addition of progesterone, wild type yeast cultures treated with H2O2 or acetate, which are both well-known PCD inducers in yeast [14][30][31][32][33][34], showed reduced ROS accumulation as measured by the ROS-driven conversion of dihydroethidium (DHE) to fluorescent ethidium (Figure 1A and B). Furthermore, under physiological culture conditions, in the absence of PCD inducers, progesterone significantly reduced ROS levels as compared to the untreated control (Figure 2A). Altogether, progesterone dampens ROS production in yeast, both in normal culture conditions and in the presence of external stress factors.
–
Progesterone impacts mitochondria by acting as a mild respiration-uncoupler
To further explore the mechanisms underlying progesterone cytoprotection, we next examined the physiology of mitochondria, since these organelles constitute one of the main sources of ROS [35][36][37][38]. Interestingly, while O2 consumption was significantly enhanced during progesterone treatment, ATP levels were reduced (Figure 2B and C). Altogether, this indicates an uncoupling phenotype with diminished oxidative phosphorylation. Accordingly, we observed reduced growth of wild type yeast upon progesterone treatment on a non-fermentable carbon source (glycerol), while no changes were detected on a fermentable carbon source (glucose) (Figure 2D and E). Importantly, this effect was also observed in a mutant strain lacking the heme-binding protein Dap1, which is the sole yeast orthologue of the human progesterone receptor (Figure 2D and E) [39]. Furthermore, we could demonstrate that stress protection by progesterone is respiration-dependent, since progesterone treatment did not confer stress resistance in respiration-deficient rho0 cells (Figure 2F). Altogether, it appears that progesterone impacts yeast mitochondrial respiration in a receptor-independent fashion.
–
Progesterone administration diminishes cytosolic Ca2+ concentrations both under physiological as well as under high calcium conditions
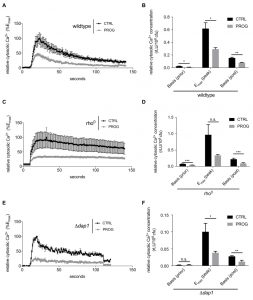
Next we investigated progesterone effects on Ca2+ homeostasis, knowing that mitochondria are one of the organelles responsible for buffering cytosolic Ca2+ under normal conditions [40]. Importantly, TBI, stroke, and even some forms of dementia cause Ca2+ accumulation in the cytosol of neurons followed by cell death and neurodegeneration [41]. Thus, we examined the capacity of yeast cells to process Ca2+ uptake under the influence of progesterone. Specifically, wild type yeast cell cultures were challenged with 150 mM CaCl2 and transient concentrations of cytoplasmic Ca2+ levels ([Ca2+]cyt) / responses were monitored. Progesterone caused a significantly reduced Ca2+ uptake capacity alongside with a faster reduction of cytoplasmic Ca2+ levels (Figure 3A and B). Of note, basal Ca2+ levels before and after the Ca2+ pulse were already lowered when cells were treated with progesterone (Figure 3B). However, mitochondrial respiration was not involved in this phenotype, since progesterone treatment continued to affect basic cytosolic Ca2+ levels in respiration-deficient rho0 cells (Figure 3C and D).
–
To further investigate the observed phenotypes, we tested single-gene deletion mutants of all currently known Ca2+ channels/transporters in yeast, including the cytoplasmic membrane transporters Cch1 and Mid 1, the organelle transporters Vcx1, Pmr1, Cod1, Yvc1, and Pmc1 as well as Emc7, an ER protein associated to Ca2+ homeostasis. Although Ca2+ uptake and clearance was influenced by some of these gene deletion, all mutants continued to exhibit significantly reduced Ca2+ uptake when treated with progesterone (compare Supplemental Figure 1A-G to H). Thus, the effects observed in wild type cells could not be reversed by single gene deletions in any of these transporters. Similarly, the effects of progesterone treatment on Ca2+ homeostasis/uptake were independent of the mammalian membrane progesterone receptor homolog Dap1 (Figure 3E and F). Taken together, progesterone seems to influence Ca2+ homeostasis/uptake in a general manner, independently from known Ca2+ transporters and respiration capacity.
DISCUSSION
Here, we establish S. cerevisiae as a model to investigate cytoprotection by progesterone. We observed that progesterone increased stress tolerance of yeast to the well-known PCD inducers H2O2 and acetate [14][30][31][32][33][34] as well as under physiological (control) conditions. Interestingly, progesterone treatment led to a mild uncoupling phenotype with higher O2 consumption (+50%) but lower ATP levels (-50%), arguing for a mitochondrial uncoupling effect. Indeed, growth on the non-fermentable carbon source glycerol was diminished in the presence of progesterone. Notably, mild uncoupling induced by chemical substances (such as dinitrophenol), caloric restriction or ectopic expression of mammalian uncoupling proteins in yeast – S. cerevisiae does not possess any known uncoupling proteins [42] – is known to increase lifespan [43][44][45]. Similarly, in mammalian aging cells, changes in mitochondrial energy metabolism caused by mitochondrial uncoupling seem to improve cellular fitness [46]. Progesterone treatment of human cells has been demonstrated to strongly increase the levels of mRNAs coding for uncoupling proteins [47]. Increased O2 consumption with decreased 32P uptake (as a parameter for ATP production) has been reported for isolated rat mitochondria treated with progesterone [48]. Collectively, our data combined with those reported in the literature highlight the possibility to investigate progesterone-mediated effects in the yeast model. The uncoupling aspect of progesterone, in fact, could represent one of the mechanisms of neuroprotection conferred by this steroid. In fact, the stress tolerance of a respiration-deficient rho0 strain was not influenced by progesterone treatment.
–
Progesterone had major effects on Ca2+ homeostasis and, in particular, on Ca2+ susceptibility/uptake. However, we could not identify any single Ca2+ channel in yeast that would influence these effects. However, we cannot exclude that yet unidentified Ca2+ channels or a combinations of known Ca2+ channels mediate these effects [49]. Another possible mode of action of progesterone on Ca2+ homeostasis could reside in its direct interaction with biological membranes. Since the chemical structure of progesterone shows four-ring as well as hydrophobic backbone and polar groups at both ends of the molecule, it could directly interact with cellular and mitochondrial membranes [50] and possibly influence their permeability towards inorganic cations (e.g. Ca2+, H+). This mode of action could connect our observations of mitochondrial uncoupling and modulation of Ca2+ homeostasis. Of note, a progesterone-treated rho0 strain still showed Ca2+ effects but no enhanced stress tolerance, suggesting that altered Ca2+ homeostasis may lie upstream of mitochondrial uncoupling. However, these mechanistic hypotheses remain to be empirically tested.
–
Certainly, the putative relevance of the herein described progesterone effects for TBI pathology remains to be explored. In some mammalian cell types, progesterone leads to a significant increase of intracellular Ca2+ [51][52], partly by activating protein kinase C [53] and depleting endocannabinoids by activating α/β hydrolase domain-containing protein 2 (ABHD2) [54]. However, in other cell types, progesterone withdrawal leads to an increased level of cytosolic Ca2+ [55]. While progesterone was not able to reduce estrogen-induced Ca2+ uptake in the rabbit myometrial smooth muscle cells, it increased the accumulation of Ca2+ in mitochondria [55]. This suggests that progesterone withdrawal reduces both myometrial cytosolic Ca2+ levels as well as the capacity of these cells to accumulate Ca2+in different cellular compartments. Similar effects were reported for other types of smooth muscles [56][57] and are believed to be caused by regulation of the inward current through L-type Ca2+ channels [56][58]. In neurons, the influence on Ca2+ signaling and the following inhibition of excitotoxic neuron death seem to be the neuroprotective mechanism induced by acute administration of progesterone after various neuronal injuries [59][60][61]. Indeed, progesterone might mediate broad neuroprotective effects, not only in the context of TBI but also in other pathologies [62][63].
–
The role of progesterone in the pathological development of TBI has been well described in recent years. It has been shown that progesterone improves the function of the blood-brain-barrier after TBI [64]. Progesterone also increases the level of circulating endothelial cells, which in turn improves neovascularization and vascular remodeling in the brain [65]. Furthermore, progesterone treatment reduces neuroinflammation and oxidative stress [66] as it improves remyelination and functional recovery [63].
–
Interestingly, the intracellular effects exerted by progesterone in our model – reduced intracellular Ca2+ levels, uncoupled mitochondria and ROS reduction – were not lost when the sole possible yeast orthologue of the human progesterone receptor was removed from the system. This suggests that progesterone mediates its broad cytoprotective effects through other proteins than steroid receptors or perhaps with cellular membrane lipids. We surmise that yeast constitutes an ideal platform for exploring these effects in further detail.
MATERIALS AND METHODS
Growth conditions
S. cerevisiae strains (Table 1) were inoculated to 5*105 (for growth curve OD600 of 0.05) cells in SC medium containing 0.17% yeast nitrogen base (BD Diagnostics; without ammonium sulfate and amino acids), 0.5% (NH4)2SO4 , 30 mg/L of all amino acids (except 80 mg/L histidine and 200 mg/L leucine), 30 mg/L adenine, and 320 mg/L uracil with 2% glucose (SCD) or alternatively with 3% glycerol (SCGly), w/o treatment with progesterone (10 µg/ml; Sigma Aldrich, Catalogue Nr. P0130). Controls were treated with respective solvent (EtOH). Where indicated, stress (acetate or H2O2) was inflicted as described previously in mid-log phase (~ 6h of growth, culture density 2-4*106 cells/ml). Due to the inherent reduced respiration-rate of BY4741 strains, TB50a strains were used for respiration-related experiments. DAP1 deletion was carried out by classical homologous recombination [67][68].
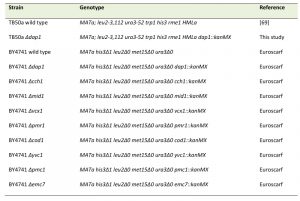 | Table 1. Strains used in this study. [69] |
–
Growth curve
Cells from ONC in SCD media were inoculated to an OD600 of 0.05 in SCD media and SCGly media with or without 10 µg/ml progesterone addition. Untreated cultures were supplemented with 0.1% EtOH for solvent control. To obtain growth curves, 300 µl of respective cultures per well were transferred into Honeycomb® plates, and measured with Bioscreen C MBR system (Oy Growth Curves Ab Ltd.) for a period of 48 hours at 28°C, using continuous shaking and OD600 measurements every 30 minutes.
–
Oxygen consumption measurement
Oxygen consumption was measured using a FireSting oxygen electrode (Pyro-Science) under constant stirring at a temperature of 28.0 ± 0.2°C in sealed 2 ml bottles. The corresponding cell counts were measured using a CASY Cell Counter, whereas percentage of living cells in the sample were established by flow cytometry with propidium iodide (PI: 100 ng/ml) stained samples. The slope of the oxygen concentration as the function of time in its linear region was calculated and normalized to the number of living cells in the sample.
–
ROS accumulation (DHE) assay
Oxidation of non-fluorescent di-hydroethidium (DHE) to fluorescent ethidium was used to measure ROS accumulation in yeast cells [38]. Approximately 5*106 cells from each sample were collected, washed and incubated with DHE solution (2.5 µg/ml in PBS) for 10 min in the dark. After washing samples were re-suspended in PBS buffer and measured using flow cytometry. The relative mean fluorescence measured for the cell population was used for analysis [69].
–
Boiling ethanol extraction of ATP and ATP measurement
ATP extraction was done with flash-frozen cells by adding 0.5 ml preheated (90°C) BES buffer and incubation at 90°C for 3 minutes. After centrifugation, supernatants were stored at -80°C until the measurement. ATP levels were determined by using the ATP detection kit from Invitrogen in a Luminoskan (Thermo Scientific).
–
Cytosolic Ca2+ measurements
[Ca2+]cyt were measured using yeast strains carrying the vector pYX212 encoding the bioluminescent protein aequorin under the control of a TPI promoter. For analysis of the cellular response to high doses of external Ca2+, an equivalent of 6*106 cells was harvested, resuspended in 200 μl SCD containing 4 μM coelenterazine and incubated for 1 h in the dark. After washing cells were measured in a Luminoskan for 10 s and then challenged with high dose of Ca2+ (pump injection of 150 mM Ca2+). Kinetics were recorded over 120 s. The luminescence signal was normalized to the OD600 of each well and reported in relative luminescence units, normalized to the global maximum value of the ethanol treated control of the respective run for better comparability.
References
- E. Baulieu, and M. Schumacher, "Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination.", Steroids, 2000. http://www.ncbi.nlm.nih.gov/pubmed/11108866
- K. Vagnerova, I.P. Koerner, and P.D. Hurn, "Gender and the Injured Brain", Anesthesia & Analgesia, vol. 107, pp. 201-214, 2008. http://dx.doi.org/10.1213/ane.0b013e31817326a5
- D. Meffre, F. Labombarda, B. Delespierre, A. Chastre, A. De Nicola, D. Stein, M. Schumacher, and R. Guennoun, "Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury", Neuroscience, vol. 231, pp. 111-124, 2013. http://dx.doi.org/10.1016/j.neuroscience.2012.11.039
- D. Si, H. Wang, Q. Wang, C. Zhang, J. Sun, Z. Wang, Z. Zhang, and Y. Zhang, "Progesterone treatment improves cognitive outcome following experimental traumatic brain injury in rats", Neuroscience Letters, vol. 553, pp. 18-23, 2013. http://dx.doi.org/10.1016/j.neulet.2013.07.052
- Z. Soltani, M. Khaksari, N. Shahrokhi, G. Mohammadi, B. Mofid, A. Vaziri, and S. Amiresmaili, "Effect of estrogen and/or progesterone administration on traumatic brain injury-caused brain edema: the changes of aquaporin-4 and interleukin-6", Journal of Physiology and Biochemistry, vol. 72, pp. 33-44, 2015. http://dx.doi.org/10.1007/s13105-015-0453-5
- H.V. Carswell, N.H. Anderson, J.S. Clark, D. Graham, B. Jeffs, A.F. Dominiczak, and I.M. Macrae, "Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat.", Hypertension (Dallas, Tex. : 1979), 1999. http://www.ncbi.nlm.nih.gov/pubmed/10024327
- N.J. Alkayed, S.J. Murphy, R.J. Traystman, P.D. Hurn, and V.M. Miller, "Neuroprotective effects of female gonadal steroids in reproductively senescent female rats.", Stroke, 2000. http://www.ncbi.nlm.nih.gov/pubmed/10625733
- G. Xiao, J. Wei, W. Yan, W. Wang, and Z. Lu, "Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial", Critical Care, vol. 12, 2008. http://dx.doi.org/10.1186/cc6887
- D.W. Wright, A.L. Kellermann, V.S. Hertzberg, P.L. Clark, M. Frankel, F.C. Goldstein, J.P. Salomone, L.L. Dent, O.A. Harris, D.S. Ander, D.W. Lowery, M.M. Patel, D.D. Denson, A.B. Gordon, M.M. Wald, S. Gupta, S.W. Hoffman, and D.G. Stein, "ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury", Annals of Emergency Medicine, vol. 49, pp. 391-402.e2, 2007. http://dx.doi.org/10.1016/j.annemergmed.2006.07.932
- B.E. Skolnick, A.I. Maas, R.K. Narayan, R.G. van der Hoop, T. MacAllister, J.D. Ward, N.R. Nelson, and N. Stocchetti, "A Clinical Trial of Progesterone for Severe Traumatic Brain Injury", New England Journal of Medicine, vol. 371, pp. 2467-2476, 2014. http://dx.doi.org/10.1056/NEJMoa1411090
- C. Lin, H. He, Z. Li, Y. Liu, H. Chao, J. Ji, and N. Liu, "Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials", Scientific Reports, vol. 5, 2015. http://dx.doi.org/10.1038/srep13442
- Y. Zeng, Y. Zhang, J. Ma, and J. Xu, "Progesterone for Acute Traumatic Brain Injury: A Systematic Review of Randomized Controlled Trials", PLOS ONE, vol. 10, pp. e0140624, 2015. http://dx.doi.org/10.1371/journal.pone.0140624
- F. Madeo, E. Fröhlich, and K.U. Fröhlich, "A yeast mutant showing diagnostic markers of early and late apoptosis.", The Journal of cell biology, 1997. http://www.ncbi.nlm.nih.gov/pubmed/9348289
- F. Madeo, E. Fröhlich, M. Ligr, M. Grey, S.J. Sigrist, D.H. Wolf, and K.U. Fröhlich, "Oxygen stress: a regulator of apoptosis in yeast.", The Journal of cell biology, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10330404
- F. Madeo, E. Herker, C. Maldener, S. Wissing, S. Lächelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K.U. Fröhlich, "A caspase-related protease regulates apoptosis in yeast.", Molecular cell, 2002. http://www.ncbi.nlm.nih.gov/pubmed/11983181
- E. Herker, H. Jungwirth, K.A. Lehmann, C. Maldener, K. Fröhlich, S. Wissing, S. Büttner, M. Fehr, S. Sigrist, and F. Madeo, "Chronological aging leads to apoptosis in yeast", The Journal of Cell Biology, vol. 164, pp. 501-507, 2004. http://dx.doi.org/10.1083/jcb.200310014
- S. Wissing, P. Ludovico, E. Herker, S. Büttner, S.M. Engelhardt, T. Decker, A. Link, A. Proksch, F. Rodrigues, M. Corte-Real, K. Fröhlich, J. Manns, C. Candé, S.J. Sigrist, G. Kroemer, and F. Madeo, "An AIF orthologue regulates apoptosis in yeast", The Journal of Cell Biology, vol. 166, pp. 969-974, 2004. http://dx.doi.org/10.1083/jcb.200404138
- S. Büttner, D. Ruli, F. Vögtle, L. Galluzzi, B. Moitzi, T. Eisenberg, O. Kepp, L. Habernig, D. Carmona-Gutierrez, P. Rockenfeller, P. Laun, M. Breitenbach, C. Khoury, K. Fröhlich, G. Rechberger, C. Meisinger, G. Kroemer, and F. Madeo, "A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis", The EMBO Journal, vol. 30, pp. 2779-2792, 2011. http://dx.doi.org/10.1038/emboj.2011.197
- L. Galluzzi, O. Kepp, and G. Kroemer, "Mitochondrial regulation of cell death: a phylogenetically conserved control", Microbial Cell, vol. 3, pp. 101-108, 2016. http://dx.doi.org/10.15698/mic2016.03.483
- S. Büttner, L. Habernig, F. Broeskamp, D. Ruli, F.N. Vögtle, M. Vlachos, F. Macchi, V. Küttner, D. Carmona-Gutierrez, T. Eisenberg, J. Ring, M. Markaki, A.A. Taskin, S. Benke, C. Ruckenstuhl, R. Braun, C. Van den Haute, T. Bammens, A. van der Perren, K. Fröhlich, J. Winderickx, G. Kroemer, V. Baekelandt, N. Tavernarakis, G.G. Kovacs, J. Dengjel, C. Meisinger, S.J. Sigrist, and F. Madeo, "Endonuclease G mediates α-synuclein cytotoxicity during Parkinson's disease", The EMBO Journal, vol. 32, pp. 3041-3054, 2013. http://dx.doi.org/10.1038/emboj.2013.228
- S. Büttner, F. Broeskamp, C. Sommer, M. Markaki, L. Habernig, A. Alavian-Ghavanini, D. Carmona-Gutierrez, T. Eisenberg, E. Michael, G. Kroemer, N. Tavernarakis, S.J. Sigrist, and F. Madeo, "Spermidine protects against α-synuclein neurotoxicity", Cell Cycle, vol. 13, pp. 3903-3908, 2014. http://dx.doi.org/10.4161/15384101.2014.973309
- J.J. Heinisch, and R. Brandt, "Signaling pathways and posttranslational modifications of tau in Alzheimer's disease: the humanization of yeast cells", Microbial Cell, vol. 3, pp. 135-146, 2016. http://dx.doi.org/10.15698/mic2016.04.489
- R. Menezes, S. Tenreiro, D. Macedo, C. Santos, and T. Outeiro, "From the baker to the bedside: yeast models of Parkinson's disease", Microbial Cell, vol. 2, pp. 262-279, 2015. http://dx.doi.org/10.15698/mic2015.08.219
- T. Amen, and D. Kaganovich, "Yeast screening platform identifies FDA-approved drugs that reduce Aβ oligomerization", Microbial Cell, vol. 3, pp. 97-100, 2016. http://dx.doi.org/10.15698/mic2016.03.482
- A. Shrestha, and L. Megeney, "Yeast proteinopathy models: a robust tool for deciphering the basis of neurodegeneration", Microbial Cell, vol. 2, pp. 458-465, 2015. http://dx.doi.org/10.15698/mic2015.12.243
- M. Bond, R. Brown, C. Rallis, J. Bahler, and S. Mole, "A central role for TOR signalling in a yeast model for juvenile CLN3 disease", Microbial Cell, vol. 2, pp. 466-480, 2015. http://dx.doi.org/10.15698/mic2015.12.241
- D. Carmona-Gutierrez, A.L. Hughes, F. Madeo, and C. Ruckenstuhl, "The crucial impact of lysosomes in aging and longevity", Ageing Research Reviews, vol. 32, pp. 2-12, 2016. http://dx.doi.org/10.1016/j.arr.2016.04.009
- R. Raghupathi, "Cell death mechanisms following traumatic brain injury.", Brain pathology (Zurich, Switzerland), 2004. http://www.ncbi.nlm.nih.gov/pubmed/15193035
- G. Cheng, R. Kong, L. Zhang, and J. Zhang, "Mitochondria in traumatic brain injury and mitochondrial‐targeted multipotential therapeutic strategies", British Journal of Pharmacology, vol. 167, pp. 699-719, 2012. http://dx.doi.org/10.1111/j.1476-5381.2012.02025.x
- P. Ludovico, M.J. Sousa, M.T. Silva, C. Leão, and M. Côrte-Real, "Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid", Microbiology, vol. 147, pp. 2409-2415, 2001. http://dx.doi.org/10.1099/00221287-147-9-2409
- O. Merkel, D. Asslaber, J. Pinon, A. Egle, and R. Greil, "Interdependent regulation of p53 and miR-34a in chronic lymphocytic leukemia", Cell Cycle, vol. 9, pp. 2836-2840, 2010. http://dx.doi.org/10.4161/cc.9.14.12267
- T. Eisenberg, D. Carmona-Gutierrez, S. Büttner, N. Tavernarakis, and F. Madeo, "Necrosis in yeast", Apoptosis, vol. 15, pp. 257-268, 2010. http://dx.doi.org/10.1007/s10495-009-0453-4
- D. Carmona-Gutiérrez, M.A. Bauer, J. Ring, H. Knauer, T. Eisenberg, S. Büttner, C. Ruckenstuhl, A. Reisenbichler, C. Magnes, G.N. Rechberger, R. Birner-Gruenberger, H. Jungwirth, K. Fröhlich, F. Sinner, G. Kroemer, and F. Madeo, "The propeptide of yeast cathepsin D inhibits programmed necrosis", Cell Death & Disease, vol. 2, pp. e161-e161, 2011. http://dx.doi.org/10.1038/cddis.2011.43
- R.J. Braun, C. Sommer, D. Carmona-Gutierrez, C.M. Khoury, J. Ring, S. Büttner, and F. Madeo, "Neurotoxic 43-kDa TAR DNA-binding Protein (TDP-43) Triggers Mitochondrion-dependent Programmed Cell Death in Yeast", Journal of Biological Chemistry, vol. 286, pp. 19958-19972, 2011. http://dx.doi.org/10.1074/jbc.M110.194852
- K.M. Holmström, and T. Finkel, "Cellular mechanisms and physiological consequences of redox-dependent signalling", Nature Reviews Molecular Cell Biology, vol. 15, pp. 411-421, 2014. http://dx.doi.org/10.1038/nrm3801
- R. Braun, C. Sommer, C. Leibiger, R. Gentier, V. Dumit, K. Paduch, T. Eisenberg, L. Habernig, G. Trausinger, C. Magnes, T. Pieber, F. Sinner, J. Dengjel, F. van Leeuwen, G. Kroemer, and F. Madeo, "Accumulation of Basic Amino Acids at Mitochondria Dictates the Cytotoxicity of Aberrant Ubiquitin", Cell Reports, vol. 10, pp. 1557-1571, 2015. http://dx.doi.org/10.1016/j.celrep.2015.02.009
- R. Braun, C. Sommer, C. Leibiger, R. Gentier, V. Dumit, K. Paduch, T. Eisenberg, L. Habernig, G. Trausinger, C. Magnes, T. Pieber, F. Sinner, J. Dengjel, F. van Leeuwen, G. Kroemer, and F. Madeo, "Modeling non-hereditary mechanisms of Alzheimer disease during apoptosis in yeast", Microbial Cell, vol. 2, pp. 136-138, 2015. http://dx.doi.org/10.15698/mic2015.04.199
- C. Ruckenstuhl, S. Büttner, D. Carmona-Gutierrez, T. Eisenberg, G. Kroemer, S.J. Sigrist, K. Fröhlich, and F. Madeo, "The Warburg Effect Suppresses Oxidative Stress Induced Apoptosis in a Yeast Model for Cancer", PLoS ONE, vol. 4, pp. e4592, 2009. http://dx.doi.org/10.1371/journal.pone.0004592
- R.A. Hand, N. Jia, M. Bard, and R.J. Craven, "Saccharomyces cerevisiae Dap1p, a novel DNA damage response protein related to the mammalian membrane-associated progesterone receptor.", Eukaryotic cell, 2003. http://www.ncbi.nlm.nih.gov/pubmed/12684380
- A. Gregor, M. Kocyłowski, and E. Kostrzewska, "[Evaluation of the diagnostic usefulness of determining porphobilinogen deaminase activity in the erythrocytes in patients with acute intermittent porphyria and in carriers of the gene of this type of porphyria].", Przeglad lekarski, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3575772
- N. Tubiana, Z. Mishal, F. le Caer, J.M. Seigneurin, Y. Berthoix, P.M. Martin, and Y. Carcassonne, "Quantification of oestradiol binding at the surface of human lymphocytes by flow cytofluorimetry.", British journal of cancer, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3489481
- D. Roussel, M. Harding, M.J. Runswick, J.E. Walker, and M.D. Brand, "Does any yeast mitochondrial carrier have a native uncoupling protein function?", Journal of bioenergetics and biomembranes, 2002. http://www.ncbi.nlm.nih.gov/pubmed/12171066
- M.H. Barros, B. Bandy, E.B. Tahara, and A.J. Kowaltowski, "Higher Respiratory Activity Decreases Mitochondrial Reactive Oxygen Release and Increases Life Span in Saccharomyces cerevisiae", Journal of Biological Chemistry, vol. 279, pp. 49883-49888, 2004. http://dx.doi.org/10.1074/jbc.M408918200
- V.P. Skulachev, "Uncoupling: new approaches to an old problem of bioenergetics", Biochimica et Biophysica Acta (BBA) - Bioenergetics, vol. 1363, pp. 100-124, 1998. http://dx.doi.org/10.1016/S0005-2728(97)00091-1
- S.A. Mookerjee, A.S. Divakaruni, M. Jastroch, and M.D. Brand, "Mitochondrial uncoupling and lifespan", Mechanisms of Ageing and Development, vol. 131, pp. 463-472, 2010. http://dx.doi.org/10.1016/j.mad.2010.03.010
- C.E. Amara, E.G. Shankland, S.A. Jubrias, D.J. Marcinek, M.J. Kushmerick, and K.E. Conley, "Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo", Proceedings of the National Academy of Sciences, vol. 104, pp. 1057-1062, 2007. http://dx.doi.org/10.1073/pnas.0610131104
- A.M. Rodriguez, M. Monjo, P. Roca, and A. Palou, "Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes.", Cellular and molecular life sciences : CMLS, 2002. http://www.ncbi.nlm.nih.gov/pubmed/12475182
- R. Wade, and H. Jones, " Effect of progesterone on mitochondrial adenosinetriphospatase.", JBC 220;547-551, 1956.
- W. Liu, "Control of Calcium in Yeast Cells", MS&A, pp. 95-122, 2012. http://dx.doi.org/10.1007/978-88-470-2490-8_5
- Z.W. Ren, "[Radiofrequency ablation of left-sided atrioventricular accessory tract to treat supraventricular tachycardia].", Zhonghua xin xue guan bing za zhi, 1992. http://www.ncbi.nlm.nih.gov/pubmed/1304487
- A. Romarowski, C. Sánchez-Cárdenas, H.V. Ramírez-Gómez, L.D.C. Puga Molina, C.L. Treviño, A. Hernández-Cruz, A. Darszon, and M.G. Buffone, "A Specific Transitory Increase in Intracellular Calcium Induced by Progesterone Promotes Acrosomal Exocytosis in Mouse Sperm1", Biology of Reproduction, vol. 94, 2016. http://dx.doi.org/10.1095/biolreprod.115.136085
- L. Li, C. Xiang, Y. Zhu, and K. Qin, "Modeling of progesterone-induced intracellular calcium signaling in human spermatozoa", Journal of Theoretical Biology, vol. 351, pp. 58-66, 2014. http://dx.doi.org/10.1016/j.jtbi.2014.02.026
- L. Bonaccorsi, "Progesterone-stimulated intracellular calcium increase in human spermatozoa is protein kinase C-independent", Molecular Human Reproduction, vol. 4, pp. 259-268, 1998. http://dx.doi.org/10.1093/molehr/4.3.259
- M.R. Miller, N. Mannowetz, A.T. Iavarone, R. Safavi, E.O. Gracheva, J.F. Smith, R.Z. Hill, D.M. Bautista, Y. Kirichok, and P.V. Lishko, "Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone", Science, vol. 352, pp. 555-559, 2016. http://dx.doi.org/10.1126/science.aad6887
- S. Batra, "Effect of estrogen and progesterone treatment on calcium uptake by the myometrium and smooth muscle of the lower urinary tract.", European journal of pharmacology, 1986. http://www.ncbi.nlm.nih.gov/pubmed/3758176
- M. Barbagallo, L.J. Dominguez, G. Licata, J. Shan, L. Bing, E. Karpinski, P.K.T. Pang, and L.M. Resnick, "Vascular Effects of Progesterone : Role of Cellular Calcium Regulation.", Hypertension (Dallas, Tex. : 1979), 2001. http://www.ncbi.nlm.nih.gov/pubmed/11208769
- Y. He, Q. Gao, B. Han, X. Zhu, D. Zhu, J. Tao, J. Chen, and Z. Xu, "Progesterone suppressed vasoconstriction in human umbilical vein via reducing calcium entry", Steroids, vol. 108, pp. 118-125, 2016. http://dx.doi.org/10.1016/j.steroids.2016.02.006
- Z. Wu, and W. Shen, "Progesterone inhibits L‐type calcium currents in gallbladder smooth muscle cells", Journal of Gastroenterology and Hepatology, vol. 25, pp. 1838-1843, 2010. http://dx.doi.org/10.1111/j.1440-1746.2010.06299.x
- J.I. Luoma, B.G. Kelley, and P.G. Mermelstein, "Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity", Steroids, 2011. http://dx.doi.org/10.1016/j.steroids.2011.02.013
- J.I. Luoma, C.M. Stern, and P.G. Mermelstein, "Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection", The Journal of Steroid Biochemistry and Molecular Biology, vol. 131, pp. 30-36, 2012. http://dx.doi.org/10.1016/j.jsbmb.2011.11.002
- W. Cai, Y. Zhu, K. Furuya, Z. Li, M. Sokabe, and L. Chen, "Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage", Neuropharmacology, vol. 55, pp. 127-138, 2008. http://dx.doi.org/10.1016/j.neuropharm.2008.04.023
- E. Brotfain, S.E. Gruenbaum, M. Boyko, R. Kutz, A. Zlotnik, and M. Klein, "Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury.", Current neuropharmacology, 2016. http://www.ncbi.nlm.nih.gov/pubmed/26955967
- J. Wei, and G. Xiao, "The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects", Acta Pharmacologica Sinica, vol. 34, pp. 1485-1490, 2013. http://dx.doi.org/10.1038/aps.2013.160
- J.L. Pascual, M.A. Murcy, S. Li, W. Gong, R. Eisenstadt, K. Kumasaka, C. Sims, D.H. Smith, K. Browne, S. Allen, and J. Baren, "Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier", The American Journal of Surgery, vol. 206, pp. 840-846, 2013. http://dx.doi.org/10.1016/j.amjsurg.2013.07.016
- Z. Li, B. Wang, Z. Kan, B. Zhang, Z. Yang, J. Chen, D. Wang, H. Wei, J. Zhang, and R. Jiang, "Progesterone Increases Circulating Endothelial Progenitor Cells and Induces Neural Regeneration after Traumatic Brain Injury in Aged Rats", Journal of Neurotrauma, vol. 29, pp. 343-353, 2012. http://dx.doi.org/10.1089/neu.2011.1807
- K.M. Webster, D.K. Wright, M. Sun, B.D. Semple, E. Ozturk, D.G. Stein, T.J. O’Brien, and S.R. Shultz, "Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury", Journal of Neuroinflammation, vol. 12, 2015. http://dx.doi.org/10.1186/s12974-015-0457-7
- U. Gueldener, J. Heinisch, G.J. Koehler, D. Voss, and J.H. Hegemann, "A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast.", Nucleic acids research, 2002. http://www.ncbi.nlm.nih.gov/pubmed/11884642
- U. Güldener, S. Heck, T. Fielder, J. Beinhauer, and J.H. Hegemann, "A new efficient gene disruption cassette for repeated use in budding yeast.", Nucleic acids research, 1996. http://www.ncbi.nlm.nih.gov/pubmed/8692690
- K. Kainz, J. Tadic, A. Zimmermann, T. Pendl, D. Carmona-Gutierrez, C. Ruckenstuhl, T. Eisenberg, and F. Madeo, "Methods to Assess Autophagy and Chronological Aging in Yeast", Methods in Enzymology, pp. 367-394, 2017. http://dx.doi.org/10.1016/bs.mie.2016.09.086
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
We thank Silvia Dichtinger for technical assistance. FM is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20, P29262, P24381, P29203 P27893, I1000 and ‘SFB Lipotox’ (F3012), as well as to BMWFW and the Karl-Franzens University for grant ‘Unkonventionelle Forschung’ and grant DKplus Metabolic and Cardiovascular Diseases (W1226). We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge”. GK is supported by the Ligue contre le Cancer Comité de Charente-Maritime (équipe labelisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); Fondation Carrefour; the LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière, the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
COPYRIGHT
© 2017

The neuroprotective steroid progesterone promotes mitochondrial uncoupling, reduces cytosolic calcium and augments stress resistance in yeast cells by
© 2017 Stekovic et al. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY) license, which allows the unrestricted use, distribution, and reproduction in any medium, provided the original author and source are acknowledged. is licensed under a Creative Commons Attribution 4.0 International License.