Research Reports:
Microbial Cell, Vol. 3, No. 3, pp. 120 - 125; doi: 10.15698/mic2016.03.485
Towards understanding the gliotoxin detoxification mechanism: in vivo thiomethylation protects yeast from gliotoxin cytotoxicity
Department of Biology, Maynooth University, Maynooth, County Kildare, Ireland.
Keywords: gliotoxin, Aspergillus fumigatus, Saccharomyces cerevisiae, oxidoreductase GliT, thio-methyltransferase GtmA.
Received originally: 09/09/2015 Received in revised form: 22/12/2015
Accepted: 07/01/2016
Published: 19/02/2016
Correspondence:
Gary W. Jones, Department of Biology, Maynooth University, Maynooth, County Kildare, Ireland gary.jones@nuim.ie
Conflict of interest statement: The authors declare no existing conflicts of interest.
Please cite this article as: Elizabeth B. Smith, Stephen K. Dolan, David A. Fitzpatrick, Sean Doyle and Gary W. Jones (2016). Towards understanding the gliotoxin detoxification mechanism: in vivo thiomethylation protects yeast from gliotoxin cytotoxicity. Microbial Cell 3(3): 120-125.
Abstract
Gliotoxin (GT) is a mycotoxin produced by some species of ascomycete fungi including the opportunistic human pathogen Aspergillus fumigatus. In order to produce GT the host organism needs to have evolved a self-protection mechanism. GT contains a redox-cycling disulfide bridge that is important in mediating toxicity. Recently is has been demonstrated that A. fumigatus possesses a novel thiomethyltransferase protein called GtmA that has the ability to thiomethylate GT in vivo, which aids the organism in regulating GT biosynthesis. It has been suggested that thiomethylation of GT and similar sulfur-containing toxins may play a role in providing self-protection in host organisms. In this work we have engineered Saccharomyces cerevisiae, a GT-naïve organism, to express A. fumigatus GtmA. We demonstrate that GtmA can readily thiomethylate GT in yeast, which results in protection of the organism from exogenous GT. Our work has implications for understanding the evolution of GT self-protection mechanisms in organisms that are GT producers and non-producers.
INTRODUCTION
Gliotoxin (GT) is a fungal natural product with known antibiotic and antifungal properties and is biosynthesized by the opportunistic human pathogen, Aspergillus fumigatus, as well as by related ascomycetes [1] [2] [3]. Gliotoxin biosynthesis is encoded by the gli gene cluster, which contains 13 genes, and the function of most of these genes has now been elucidated [4]. Effectively, GT is the prototypic epipolythiodioxopiperazine (ETP) and is structurally and functionally related to a range of disulfide bridge-containing non-ribosomal peptides including, amongst others, sporidesmin A and sirodesmin [2] [5]. GT is redox-active; thus, cycling between the oxidized and reduced dithiol form (GT-(SH)2) can generate reactive oxygen species, which have deleterious cellular effects. GT auto-induces its own biosynthesis by activating and maintaining gli cluster expression [6]. In animal cells, GT can inactivate selected protein functionality by covalent interaction with protein thiols and also deplete cellular glutathione (GSH) [7] [8]. Indeed, the cytotoxicity of GT is such that an independently regulated gene within the gli cluster, gliT encodes GT oxidoreductase which recognizes GT-(SH)2 and catalyzes disulfide bridge closure and is essential in A. fumigatus for self-protection against exogenous GT [9] [10] [11].
–
GT is not biosynthesized by Saccharomyces cerevisiae (baker’s yeast). As a consequence, no endogenous protection system against GT exists in this species and S. cerevisiae growth can be inhibited by exposure to GT [10] [12] [13]. Expression of the A. fumigatus gliT-mediated self-protection system in yeast has been shown to confer GT resistance, as has deletion of GSH1, responsible for GSH biosynthesis [10] [12]. Thus, by enabling disulfide bridge closure, preventing the GSH-mediated chemical reduction of exogenously-added GT to GT-(SH)2 or facilitating GT efflux, the resistance of gliotoxin-naïve species to this redox-active molecular species can be augmented.
–
Gliotoxin bis-thiomethyltransferase (GtmA), the first bis thiomethyltransferase so far identified in any species, has recently been described in A. fumigatus and it has been shown to convert GT-(SH)2 to bisdethiobis(methylthio)gliotoxin (BmGT) (Figure 1) [14]. GtmA has also been contemporaneously characterized by others, where it is referred to as TmtA [15]. Interestingly, although GtmA is encoded outside the gli cluster, its expression is induced by GT and it has been convincingly demonstrated to dissipate GT levels thereby attenuating gli cluster activity and concomitant GT biosynthesis [14]. In effect, GtmA appears to be the ‘off-switch’ for GT biosynthesis in A. fumigatus. Although an unidentified thiomethyltransferase has been shown to confer resistance to a bacterial non-ribosomal peptide, holomycin, in Streptomyces clavivulgaris [16], gtmA deletion has effectively no impact on the sensitivity of A. fumigatus to GT [14] [15].
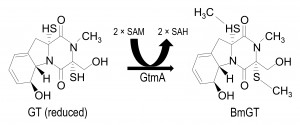 | FIGURE 1: Conversion of reduced GT to BmGT is mediated by GtmA protein. Reduced GT undergoes bis-thiomethylation via the action of GtmA protein and requires two molecules of SAM for the reaction to reach completion [14]. Consequently two molecules of SAH are produced that re-enter the methyl-methionine cycle [17]. |
–
Expression of gtmA in S. cerevisiae was therefore undertaken to further investigate the functionality of this enzyme and to explore the hypothesis that, in the absence of concomitant GT biosynthesis and a gliT-mediated self-protection system, GtmA could confer resistance to GT via BmGT formation.
RESULTS AND DISCUSSION
Expression of gtmA in yeast increases resistance to gliotoxin
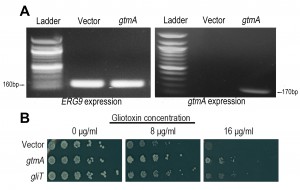
While it has been clearly demonstrated that GliT provides the primary self-protection mechanism against GT in A. fumigatus [9] [10] and that the production of BmGT via GtmA is primarily linked to GT biosynthesis and regulation [14], given that the production of BmGT will nullify GT ability to redox cycle, we hypothesized that under certain biological conditions GtmA may be able to provide direct protection against GT toxicity. In previous work from our group and others [10] [12] [13] yeast was utilized as a tool to explore the in vivo effects of exposure to GT. Following cloning of gtmA under control of the constitutive SSA2 promoter we demonstrated that gtmA is well expressed in yeast (Figure 2A). Growth of yeast is severely impaired on medium containing gliotoxin (8 and 16 μg/mL, respectively), but expression of gtmA in yeast provides protection against this cytotoxicity (Figure 2B). The level of protection provided to yeast by gtmA is more prominent than that provided by gliT expression (Figure 2B). Additionally, the level of protection provided when both gliT and gtmA are expressed in yeast is comparable to gtmA expression alone, which reflects the apparent more efficient GT detoxification of GtmA compared to GliT when engineered in yeast. The expression of both gtmA and gliT does not allow yeast to efficiently grow on GT concentration above those used in this study [data not show]. Expression of the other methyltransferases encoded within the gli-cluster, gliM and gliN [15], did not provide any protection in yeast against GT toxicity (data not shown).
–
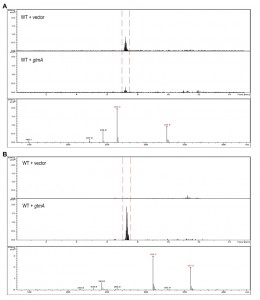
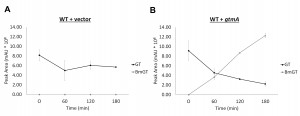
Expression of gtmA in yeast causes BmGT formation from exogenously-added gliotoxin
Yeast does not contain an ortholog or homolog of A. fumigatus gtmA [14]. Consequently it is not surprising that we have never detected the production of BmGT in yeast following exposure to GT (Figure 3A and Figure 4A). The expression of gtmA in yeast provides this GT-naïve organism with the capability of converting GT to BmGT (Figure 3B). The production of BmGT in yeast expressing gtmA is as a result of utilizing exogenous GT as a substrate (Figure 4B).
–
Biological relevance for gliotoxin conversion to BmGT
What is the biological significance for GT to BmGT conversion? The results we present here clearly show that GT conversion to BmGT protects against cytotoxicity and therefore appear to support the suggestion that thiomethylation of GT is a protection mechanism, perhaps from exogenous and/or endogenously produced GT. However, to uncover the true biological relevance of GT to BmGT conversion we need to consider this reaction in the context of organisms that produce GT and those that do not. Recent evidence has clearly shown that deletion of gtmA, and hence the removal of the ability to convert GT to BmGT, in A. fumigatus does not produce a GT-sensitive phenotype [14] [15]. This is due to the maintenance of a fully functional, primary detoxification system, GliT, in a gtmA deletion strain. Furthermore, it has recently been demonstrated that GliT functions in conjunction with the GT-specific transporter GliA to maintain intracellular GT at a level that is not toxic to the host cell [17]. The production of BmGT in A. fumigatus facilitates the secretion of this product in a GliA independent manner [17]. The secretion of BmGT from fungal cells most likely occurs through a non-specific mechanism as the molecule is efficiently secreted from yeast (this study) and from GliA-deficient A. fumigatus [17]. Thus the primary physiological relevance of converting GT to BmGT in GT-producing organisms appears to be as a negative regulator of GT biosynthesis, followed by secretion of the inactive thiomethylated derivative [17]. However, the in vivo consequence of GtmA activity, and consequent BmGT production and secretion, will depend upon whether an organism is a GT producer or not.
–
Here we clearly show that engineering a GT to BmGT conversion mechanism into a GT naïve organism, such as yeast, can provide efficient protection against exogenous GT (Figures 2, 3 and 4). The yeast genome does not encode for any GtmA homologs and does not endogenously convert GT to BmGT (Figure 3). Previous phylogenetic analysis identified organisms within the fungal kingdom that possess GtmA homologs [14] and the implications from the results of this study suggest that a GT non-producing organism that possesses a GtmA homolog may well utilize such a protein as a defense mechanism against GT exposure. The absence of GT production in such organisms may suggest that from an evolutionary perspective such GtmA homologs may have evolved to carry out other GT-independent functions in the cell and, if they possess the ability to produce BmGT, this may not be the primary function of the protein. Conversely, GT non-producing fungi may have retained GtmA homologs as a means of occupying the same habitats as GT-producing fungi.
–
Moreover, the possibility does exist that GtmA homologs in GT naïve fungi constitute an ancient ETP generic-defense mechanism that has allowed the acquisition and development of the GT-producing gene cluster, or other ETP clusters. The evolution of the primary detoxification system involving GliT and GliA, in conjunction with the presence of GtmA, has then allowed for intricate systems-level interactions to develop in organisms such as A. fumigatus, as recently shown for GT biosynthesis and interplay with the methyl/methionine cycle [17] and for regulation of the gli-cluster itself [14]. Any effect upon GT sensitivity of removing the GT to BmGT conversion system in A. fumigatus, or any GT-producing organism, may only become apparent when undertaken along with the concomitant abrogation of the primary GT-protection mechanism from the organism. Support for this hypothesis comes from the recent discovery that deletion of the gtmA homolog in GT-naïve Aspergillus niger results in sensitivity to exogenously added GT [18].
MATERIALS AND METHODS
Yeast strains, plasmids and genetic methods
The S. cerevisiae strain used in this study was BY4741 (MATa; his3Δ 1; leu2Δ 0; met15Δ 0; ura3Δ 0) and was obtained from Euroscarf. All media used were as previously described by [19]. Cultures were grown at 30°C with shaking at 200 rpm.
–
To observe the effects of gtmA expression in S. cerevisiae, gtmA was amplified from A. fumigatus cDNA (ATCC26933) using primers gtmA F and gtmA R (Table 1) and cloned into the yeast shuttle vector pC210 as previously described by [10]. Briefly, primers were designed that incorporated NdeI and SphI restriction sites. Following PCR amplification of the gtmA gene, digestion of the gtmA PCR product and of pC210 was carried out followed by ligation using T4 DNA ligases (Promega) according to manufacturer’s instructions to create pC210-gtmA. Cloning of the gtmA fragment and construct were confirmed using Sanger sequencing.
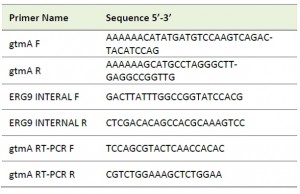 | TABLE 1. Oligonucleotide primers used in this study. |
–
Growth analysis was carried out by diluting an overnight culture of cells in fresh medium lacking leucine (-LEU) to an OD600 = 0.2 and incubated at 30°C shaking 200 rpm till an OD600 = 0.4 was reached. Cells were transferred to a microtiter plate and serially diluted. Cells were transferred to –LEU plates containing the desired concentration of GT (Sigma) using a replicator and incubated at 30°C for 48 h, with further monitoring at room temperature for 72 h.
–
RT-PCR
Total RNA was extracted from 5 mL cultures of the yeast strains grown overnight at 30°C shaking 200 rpm. RNA was extracted using the QIAGEN RNeasy plant mini kit as per manufacturers’ guidelines. RNA was DNase treated using DNase I kit (Sigma-Aldrich), according to the manufacturer’s recommendations. RNA concentrations were measured using a Nano Drop 1000 Spectrophotometer (Mason Technology).
–
cDNA was synthesized using the qScriptTM cDNA Supermix (Quanta Biosciences) as per manufacturer’s instructions. AccuTaq LA polymerase (Sigma) was used to amplify a housekeeping gene ERG9 as a control and the gene of interest gtmA. The primers used in RT-PCR reactions are listed in Table 1. PCR reactions were conducted in a PTC-200 Peltier Thermal Cycler (MJ Research) and the program consisted of 1 cycle of 95°C for 5 min, 35 cycles of 95°C for 30 sec, 58°C for 30 sec and 68°C for 30 sec. Followed by a further cycle of 68°C for 10 min.
–
Mass spectrometry detection of gliotoxin and bis-methylgliotoxin
GT uptake and BmGT conversion assays were carried out by diluting overnight cell cultures in -LEU medium to an OD600 = 0.4 and incubation at 30°C shaking 200 rpm till an OD600 = 1.2 was reached, followed by GT (5 μg/ml) addition and incubation for a further 3 h. Samples were taken every 30 min during the 3 h period, and were then centrifuged at 5,000 x g to obtain the supernatant, which was then organically extracted using an equal volume of chloroform. Organic extracts were dried to completion under a vacuum and re-solubilized in methanol and analyzed by LC-MS (Agilent LC-MS system- Model 6340) as previously described [20].
References
- J.R. Johnson, W.F. Bruce, and J.D. Dutcher, "Gliotoxin, The Antibiotic Principle of Gliocladium fimbriatum. I. Production, Physical and Biological Properties1", Journal of the American Chemical Society, vol. 65, pp. 2005-2009, 1943. http://dx.doi.org/10.1021/ja01250a051
- D.M. Gardiner, P. Waring, and B.J. Howlett, "The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis", Microbiology, vol. 151, pp. 1021-1032, 2005. http://dx.doi.org/10.1099/mic.0.27847-0
- J.J. Coleman, S. Ghosh, I. Okoli, and E. Mylonakis, "Antifungal Activity of Microbial Secondary Metabolites", PLoS ONE, vol. 6, pp. e25321, 2011. http://dx.doi.org/10.1371/journal.pone.0025321
- D.M. Gardiner, and B.J. Howlett, "Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster ofAspergillus fumigatus", FEMS Microbiology Letters, vol. 248, pp. 241-248, 2005. http://dx.doi.org/10.1016/j.femsle.2005.05.046
- D.M. Gardiner, A.J. Cozijnsen, L.M. Wilson, M.S.C. Pedras, and B.J. Howlett, "The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans", Molecular Microbiology, vol. 53, pp. 1307-1318, 2004. http://dx.doi.org/10.1111/j.1365-2958.2004.04215.x
- G. O’Keeffe, S. Hammel, R.A. Owens, T.M. Keane, D.A. Fitzpatrick, G.W. Jones, and S. Doyle, "RNA-seq reveals the pan-transcriptomic impact of attenuating the gliotoxin self-protection mechanism in Aspergillus fumigatus", BMC Genomics, vol. 15, 2014. http://dx.doi.org/10.1186/1471-2164-15-894
- P.H. Bernardo, N. Brasch, C.L. Chai, and P. Waring, "A Novel Redox Mechanism for the Glutathione-dependent Reversible Uptake of a Fungal Toxin in Cells", Journal of Biological Chemistry, vol. 278, pp. 46549-46555, 2003. http://dx.doi.org/10.1074/jbc.M304825200
- P. Waring, and J. Beaver, "Gliotoxin and related epipolythiodioxopiperazines", General Pharmacology: The Vascular System, vol. 27, pp. 1311-1316, 1996. http://dx.doi.org/10.1016/S0306-3623(96)00083-3
- D.H. Scharf, N. Remme, T. Heinekamp, P. Hortschansky, A.A. Brakhage, and C. Hertweck, "Transannular Disulfide Formation in Gliotoxin Biosynthesis and Its Role in Self-Resistance of the Human Pathogen Aspergillus fumigatus", Journal of the American Chemical Society, vol. 132, pp. 10136-10141, 2010. http://dx.doi.org/10.1021/ja103262m
- M. Schrettl, S. Carberry, K. Kavanagh, H. Haas, G.W. Jones, J. O'Brien, A. Nolan, J. Stephens, O. Fenelon, and S. Doyle, "Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Aspergillus fumigatus Against Exogenous Gliotoxin", PLoS Pathogens, vol. 6, pp. e1000952, 2010. http://dx.doi.org/10.1371/journal.ppat.1000952
- S.K. Dolan, G. O’Keeffe, G.W. Jones, and S. Doyle, "Resistance is not futile: gliotoxin biosynthesis, functionality and utility", Trends in Microbiology, vol. 23, pp. 419-428, 2015. http://dx.doi.org/10.1016/j.tim.2015.02.005
- S. Carberry, E. Molloy, S. Hammel, G. O’Keeffe, G.W. Jones, K. Kavanagh, and S. Doyle, "Gliotoxin effects on fungal growth: Mechanisms and exploitation", Fungal Genetics and Biology, vol. 49, pp. 302-312, 2012. http://dx.doi.org/10.1016/j.fgb.2012.02.003
- G. Chamilos, R.E. Lewis, G.A. Lamaris, N.D. Albert, and D.P. Kontoyiannis, "Genomewide Screening for Genes Associated with Gliotoxin Resistance and Sensitivity in Saccharomyces cerevisiae", Antimicrobial Agents and Chemotherapy, vol. 52, pp. 1325-1329, 2008. http://dx.doi.org/10.1128/AAC.01393-07
- S. Dolan, R. Owens, G. O’Keeffe, S. Hammel, D. Fitzpatrick, G. Jones, and S. Doyle, "Regulation of Nonribosomal Peptide Synthesis: bis-Thiomethylation Attenuates Gliotoxin Biosynthesis in Aspergillus fumigatus", Chemistry & Biology, vol. 21, pp. 999-1012, 2014. http://dx.doi.org/10.1016/j.chembiol.2014.07.006
- D.H. Scharf, A. Habel, T. Heinekamp, A.A. Brakhage, and C. Hertweck, "Opposed Effects of Enzymatic Gliotoxin N- and S-Methylations", Journal of the American Chemical Society, vol. 136, pp. 11674-11679, 2014. http://dx.doi.org/10.1021/ja5033106
- B. Li, R.R. Forseth, A.A. Bowers, F.C. Schroeder, and C.T. Walsh, "A Backup Plan for Self‐Protection: S‐Methylation of Holomycin Biosynthetic Intermediates in Streptomyces clavuligerus", ChemBioChem, vol. 13, pp. 2521-2526, 2012. http://dx.doi.org/10.1002/cbic.201200536
- R.A. Owens, G. O'Keeffe, E.B. Smith, S.K. Dolan, S. Hammel, K.J. Sheridan, D.A. Fitzpatrick, T.M. Keane, G.W. Jones, and S. Doyle, "Interplay between Gliotoxin Resistance, Secretion, and the Methyl/Methionine Cycle in Aspergillus fumigatus", Eukaryotic Cell, vol. 14, pp. 941-957, 2015. http://dx.doi.org/10.1128/EC.00055-15
- L. Manzanares-Miralles, �. Sarikaya-Bayram, E.B. Smith, S.K. Dolan, �. Bayram, G.W. Jones, and S. Doyle, "Quantitative proteomics reveals the mechanism and consequence of gliotoxin-mediated dysregulation of the methionine cycle in Aspergillus niger", Journal of Proteomics, vol. 131, pp. 149-162, 2016. http://dx.doi.org/10.1016/j.jprot.2015.10.024
- H.M. Loovers, E. Guinan, and G.W. Jones, "Importance of the Hsp70 ATPase Domain in Yeast Prion Propagation", Genetics, vol. 175, pp. 621-630, 2007. http://dx.doi.org/10.1534/genetics.106.066019
- R.A. Owens, S. Hammel, K.J. Sheridan, G.W. Jones, and S. Doyle, "A Proteomic Approach to Investigating Gene Cluster Expression and Secondary Metabolite Functionality in Aspergillus fumigatus", PLoS ONE, vol. 9, pp. e106942, 2014. http://dx.doi.org/10.1371/journal.pone.0106942
ACKNOWLEDGMENTS
This work was funded by a Science Founda-tion Ireland Principal Investigator Award to SD and collabora-tor GWJ (PI/11/1188). GWJ has been supported by Science Foundation Ireland grant RFP08/BMT/1439. SKD is a recipient of an Irish Research Council Embark PhD Fellowship. LC-MS facilities were funded by the Irish Higher Education Authority.
COPYRIGHT
© 2016

Towards understanding the gliotoxin detoxification mechanism: in vivo thiomethylation protects yeast from gliotoxin cytotoxicity by Smith et al. is licensed under a Creative Commons Attribution 4.0 International License.