News and thoughts:
Microbial Cell, Vol. 4, No. 5, pp. 140 - 143; doi: 10.15698/mic2017.05.571
When a ribosomal protein grows up – the ribosome assembly path of Rps3
Institute of Molecular Biosciences, University of Graz, Graz, Austria.
Keywords: ribosomal protein, ribosomal RNA, ribosome biogenesis, chaperone, nuclear import, Rps3.
Received originally: 08/03/2017 Accepted: 09/03/2017
Published: 27/03/2017
Correspondence:
Brigitte Pertschy, brigitte.pertschy@uni-graz.at
Conflict of interest statement:
The author states that she has no conflict of interests.
Please cite this article as: Brigitte Pertschy (2017). When a ribosomal protein grows up - the ribosome assembly path of Rps3. Microbial Cell 4(5): 140-143. doi: 10.15698/mic2017.05.571
The biogenesis of ribosomes is a central process in all dividing cells. Eukaryotic ribosomes are composed of a large 60S and a small 40S subunit, each comprising a complex assembly of ribosomal RNA (rRNA) and ribosomal proteins (r-proteins). The synthesis of these constituents is spatially separated, with r-proteins being produced by translation in the cytoplasm, while rRNA is generated by transcription in the nucleus. Hence, the arrangement of r-proteins and rRNA into large ribonucleoprotein complexes requires dedicated mechanisms ensuring their encounter in the same compartment. To this end, r-proteins need to be safely delivered to the nucleus where they assemble with the rRNA. Beyond these initial challenges, the synthesis of ribosomes does not merely comprise the joining of r-proteins with rRNA, but occurs in a complex assembly line involving multiple maturation steps, including the processing and folding of rRNA. R-proteins usually have composite rRNA binding sites, with several different rRNA helices contributing to the full interaction. Not all of these interaction sites may already be accessible at the point when an r-protein is incorporated, necessitating that some of the r-protein-rRNA contacts are formed at later maturation stages. In our two recent studies, we investigated the ribosome assembly path of r-proteins in the yeast Saccharomyces cerevisiae using the small subunit r-protein S3 (Rps3) as a model. Our studies revealed intricate mechanisms to protect the protein, transport it into the nucleus, integrate it into pre-ribosomal precursor particles and promote its final stable association with 40S subunits.
–
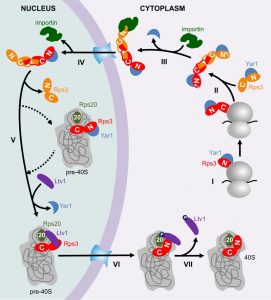
To familiarize you with these mechanisms, I invite you to join in with me in following the early life of Rps3 from its birth, through its cumbersome journey to the nucleus, its constricted initial allocation in pre-40S particles, until its conquest of a stable position in the 40S subunit (also shown in the model in Figure 1).
Like most r-proteins, free Rps3 (outside its “natural” context in the ribosome) is prone to aggregation, presumably due to its high content in positive charges, which causes non-specific interactions with RNA [1]. To prevent such undesired interactions, Rps3’s delicate regions are shielded by two mechanisms. Firstly, already while it is being synthesized, Rps3’s N-terminus is bound by a specific, “dedicated” chaperone, the ankyrin repeat protein Yar1 (Figure 1, Step I) [1][2]. Secondly, Rps3’s C-terminus dimerizes with a second Rps3 molecule by domain swapping of two beta-strands (Figure 1, Step II) [3][4].
–
Surprisingly, we found that in vivo only one of the Rps3s within the dimer is bound by Yar1 [3]. A potential explanation for this unexpected architecture comes from the observation that the Yar1 binding site is positioned directly adjacent to the nuclear localization signal (NLS) of Rps3 [5]. NLSs comprise the binding sites for importins, which mediate the transport of proteins through the nuclear pores into the nucleus [6]. Our analyses suggest that several redundant routes engage in Rps3 import, with major contribution from the classical importin α/importin β pathway [5]. We found that binding of importin α (Kap60 in yeast) competed with Yar1 for binding to Rps3, suggesting that one N-domain of Rps3 can only bind either importin or Yar1 (Figure 1, Step III). As we nevertheless detected complexes containing Rps3 and both Yar1 and Kap60 in vivo, Rps3-dimers with Yar1 bound to one and importin bound to the second Rps3 N-domain might represent the preferred conformation for nuclear import [5]. It is still a puzzle how such architecture is retained, but assuming that the complex is imported immediately after one of the two Yar1 copies has been replaced by importin, the limited time for a second importin to access the second Rps3 copy might make it simply more likely that one Yar1 is maintained. After nuclear import and dissociation of importin (Figure 1, Step IV), Rps3 is incorporated into pre-40S particles, permitting that the two protective mechanisms for Rps3 transport are dismissed again by resolving Rps3 dimers and releasing Yar1 (Figure 1, Step V). Yar1 release is presumably promoted by binding of a new partner to the Rps3 N-domain, the ribosome assembly factor Ltv1 [3]; this may happen either before, upon, or shortly after Rps3 incorporation. It is not known how exactly Rps3 dimers are disassembled, but potentially, integration of one Rps3 sterically constraints the second Rps3, which might consequently be competed away. Although the exact positioning of Rps3 within these pre-40S particles is not known, Rps3 definitely cannot be assembled in its mature conformation, considering that Ltv1 not only associates with the Rps3 N-domain, but also occupies the rRNA site, which is entitled to the Rps3 N-domain in mature 40S subunits [3][7][8]. We observed that the Rps3 N-domain alone fused to GFP displays a nuclear localization, suggesting it cannot be integrated into pre-40S particles and co-exported into the cytoplasm; consequently, the Rps3 C-domain may comprise the main anchorage of Rps3 within pre-40S particles [5].
–
Last but not least, after pre-40S export, Ltv1 has to be removed in order that Rps3 can move into its correct position [3]. At least two different events are critical for Ltv1 release, the order of which is not yet clear (Figure 1, Step VI); (i) the formation of contacts between the Rps3 N-domain and another ribosomal protein, Rps20, which might compete Ltv1 away from one of its rRNA binding sites [3] and (ii) phosphorylation of Ltv1 by the kinase Hrr25, which presumably causes electrostatic repulsion [3][9][10]. Together, these events cumulate in the release of Ltv1, allowing for the final, stable incorporation of Rps3 into 40S subunits (Figure 1, Step VII).
–
Together, our two studies revealed an unexpected complexity of the ribosome assembly path of Rps3. Although the details will certainly vary between different r-proteins, similar mechanisms as utilized by Rps3 may also be employed by other r-proteins. The engagement of dedicated chaperones was also reported for some other r-proteins in recent years, and more dedicated r-protein chaperones might still await their discovery [2][11][12][13][14][15][16][17][18][19][20][21].
–
An intriguing observation in our study was the dimerization of Rps3, which we suppose functions in protecting the C-terminal domain of Rps3 from non-specific interactions. Another r-protein, Asc1, was also reported to form dimers [22]. Hence, dimerization might be a universal strategy also used by other r-proteins to shield important sites from non-specific interactions.
–
Last but not least, we characterized a mechanism ensuring the controlled stepwise incorporation of an r-protein by means of selective occlusion of rRNA binding sites by an assembly factor. This might provide the necessary time to sculpture the respective region before it encounters its r-protein binding partner. Subsequently, the respective assembly factor must be released in order to allow the r-protein to form its contacts with the initially withholded sites. Considering that in the course of ribosome maturation, ~250 non-ribosomal proteins bind pre-ribosomal particles at different stages in the pathway, it is likely that, analogously to Ltv1, also other assembly factors impede the full incorporation of r-proteins and that assembly factor release is connected to the stable integration of the respective r-proteins.
–
The investigation of the particular mechanisms utilized in the assembly paths of other r-proteins will be an interesting subject for future studies.
References
- B. Koch, V. Mitterer, J. Niederhauser, T. Stanborough, G. Murat, G. Rechberger, H. Bergler, D. Kressler, and B. Pertschy, "Yar1 Protects the Ribosomal Protein Rps3 from Aggregation", Journal of Biological Chemistry, vol. 287, pp. 21806-21815, 2012. http://dx.doi.org/10.1074/jbc.M112.365791
- P. Pausch, U. Singh, Y.L. Ahmed, B. Pillet, G. Murat, F. Altegoer, G. Stier, M. Thoms, E. Hurt, I. Sinning, G. Bange, and D. Kressler, "Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones", Nature Communications, vol. 6, 2015. http://dx.doi.org/10.1038/ncomms8494
- V. Mitterer, G. Murat, S. Réty, M. Blaud, L. Delbos, T. Stanborough, H. Bergler, N. Leulliot, D. Kressler, and B. Pertschy, "Sequential domain assembly of ribosomal protein S3 drives 40S subunit maturation", Nature Communications, vol. 7, 2016. http://dx.doi.org/10.1038/ncomms10336
- S. Holzer, N. Ban, and S. Klinge, "Crystal Structure of the Yeast Ribosomal Protein rpS3 in Complex with Its Chaperone Yar1", Journal of Molecular Biology, vol. 425, pp. 4154-4160, 2013. http://dx.doi.org/10.1016/j.jmb.2013.08.022
- V. Mitterer, N. Gantenbein, R. Birner-Gruenberger, G. Murat, H. Bergler, D. Kressler, and B. Pertschy, "Nuclear import of dimerized ribosomal protein Rps3 in complex with its chaperone Yar1", Scientific Reports, vol. 6, 2016. http://dx.doi.org/10.1038/srep36714
- Y.M. Chook, and K.E. Süel, "Nuclear import by karyopherin-βs: Recognition and inhibition", Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, vol. 1813, pp. 1593-1606, 2011. http://dx.doi.org/10.1016/j.bbamcr.2010.10.014
- S. Granneman, E. Petfalski, A. Swiatkowska, and D. Tollervey, "Cracking pre-40S ribosomal subunit structure by systematic analyses of RNA–protein cross-linking", The EMBO Journal, vol. 29, pp. 2026-2036, 2010. http://dx.doi.org/10.1038/emboj.2010.86
- A. Ben-Shem, N. Garreau de Loubresse, S. Melnikov, L. Jenner, G. Yusupova, and M. Yusupov, "The Structure of the Eukaryotic Ribosome at 3.0 Å Resolution", Science, vol. 334, pp. 1524-1529, 2011. http://dx.doi.org/10.1126/science.1212642
- T. Schäfer, B. Maco, E. Petfalski, D. Tollervey, B. Böttcher, U. Aebi, and E. Hurt, "Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit", Nature, vol. 441, pp. 651-655, 2006. http://dx.doi.org/10.1038/nature04840
- H. Ghalei, F.X. Schaub, J.R. Doherty, Y. Noguchi, W.R. Roush, J.L. Cleveland, M.E. Stroupe, and K. Karbstein, "Hrr25/CK1δ-directed release of Ltv1 from pre-40S ribosomes is necessary for ribosome assembly and cell growth", Journal of Cell Biology, vol. 208, pp. 745-759, 2015. http://dx.doi.org/10.1083/jcb.201409056
- B. Pillet, V. Mitterer, D. Kressler, and B. Pertschy, "Hold on to your friends: Dedicated chaperones of ribosomal proteins", BioEssays, vol. 39, pp. 1-12, 2016. http://dx.doi.org/10.1002/bies.201600153
- S. Schaper, M. Fromont-Racine, P. Linder, J. de la Cruz, A. Namane, and M. Yaniv, "A yeast homolog of chromatin assembly factor 1 is involved in early ribosome assembly", Current Biology, vol. 11, pp. 1885-1890, 2001. http://dx.doi.org/10.1016/s0960-9822(01)00584-x
- T.L. Iouk, J.D. Aitchison, S. Maguire, and R.W. Wozniak, "Rrb1p, a Yeast Nuclear WD-Repeat Protein Involved in the Regulation of Ribosome Biosynthesis", Molecular and Cellular Biology, vol. 21, pp. 1260-1271, 2001. http://dx.doi.org/10.1128/MCB.21.4.1260-1271.2001
- M. West, J.B. Hedges, A. Chen, and A.W. Johnson, "Defining the Order in Which Nmd3p and Rpl10p Load onto Nascent 60S Ribosomal Subunits", Molecular and Cellular Biology, vol. 25, pp. 3802-3813, 2005. http://dx.doi.org/10.1128/MCB.25.9.3802-3813.2005
- D.P. Eisinger, F.A. Dick, E. Denke, and B.L. Trumpower, "SQT1, Which Encodes an Essential WD Domain Protein of Saccharomyces cerevisiae, Suppresses Dominant-Negative Mutations of the Ribosomal Protein Gene QSR1", Molecular and Cellular Biology, vol. 17, pp. 5146-5155, 1997. http://dx.doi.org/10.1128/mcb.17.9.5146
- Y. Ting, T. Lu, A.W. Johnson, J. Shie, B. Chen, S. Kumar S., and K. Lo, "Bcp1 Is the Nuclear Chaperone of Rpl23 in Saccharomyces cerevisiae", Journal of Biological Chemistry, vol. 292, pp. 585-596, 2017. http://dx.doi.org/10.1074/jbc.M116.747634
- S. Schütz, U. Fischer, M. Altvater, P. Nerurkar, C. Peña, M. Gerber, Y. Chang, S. Caesar, O.T. Schubert, G. Schlenstedt, and V.G. Panse, "A RanGTP-independent mechanism allows ribosomal protein nuclear import for ribosome assembly", eLife, vol. 3, 2014. http://dx.doi.org/10.7554/eLife.03473
- C. Peña, S. Schütz, U. Fischer, Y. Chang, and V.G. Panse, "Prefabrication of a ribosomal protein subcomplex essential for eukaryotic ribosome formation", eLife, vol. 5, 2016. http://dx.doi.org/10.7554/eLife.21755
- B. Pillet, J.J. García-Gómez, P. Pausch, L. Falquet, G. Bange, J. de la Cruz, and D. Kressler, "The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site", PLOS Genetics, vol. 11, pp. e1005565, 2015. http://dx.doi.org/10.1371/journal.pgen.1005565
- D. Kressler, G. Bange, Y. Ogawa, G. Stjepanovic, B. Bradatsch, D. Pratte, S. Amlacher, D. Strauß, Y. Yoneda, J. Katahira, I. Sinning, and E. Hurt, "Synchronizing Nuclear Import of Ribosomal Proteins with Ribosome Assembly", Science, vol. 338, pp. 666-671, 2012. http://dx.doi.org/10.1126/science.1226960
- P. Stelter, F. Huber, R. Kunze, D. Flemming, A. Hoelz, and E. Hurt, "Coordinated Ribosomal L4 Protein Assembly into the Pre-Ribosome Is Regulated by Its Eukaryote-Specific Extension", Molecular Cell, vol. 58, pp. 854-862, 2015. http://dx.doi.org/10.1016/j.molcel.2015.03.029
- L. Yatime, K.L. Hein, J. Nilsson, and P. Nissen, "Structure of the RACK1 Dimer from Saccharomyces cerevisiae", Journal of Molecular Biology, vol. 411, pp. 486-498, 2011. http://dx.doi.org/10.1016/j.jmb.2011.06.017
ACKNOWLEDGMENTS
This work was supported by grants P27996-B21 and P28874-B21 from the Austrian Science Fund (FWF) to Brigitte Pertschy.
COPYRIGHT
© 2017

When a ribosomal protein grows up – the ribosome assembly path of Rps3 by Brigitte Pertschy is licensed under a Creative Commons Attribution 4.0 International License.