Back to article: pH homeostasis links the nutrient sensing PKA/TORC1/Sch9 ménage-à-trois to stress tolerance and longevity
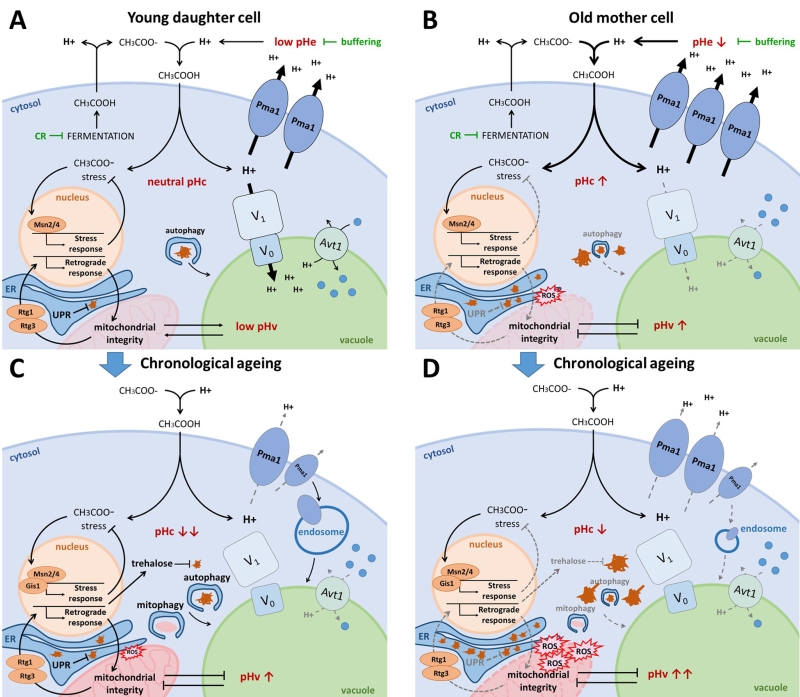
FIGURE 3: The interplay between pH control and ageing. (A) Young daughter cell. Fermentatively growing cells produce acetic acid, which is extruded from the cell. When protonated in the acidic medium, acetic acid passes the plasma membrane causing cytosolic acidification and acetic acid stress. CR and medium buffering counteract this phenomenon by reducing fermentation and protonation of acetic acid respectively. The optimally active Pma1 and V-ATPase retain the cytosolic pH around neutrality by counteracting this proton influx. The resulting acidic vacuole allows for optimal amino acid uptake by the proton/amino acid antiporter Avt1 and efficient autophagy. Acetic acid stress induces the environmental stress response aided by transcription factors Msn2/4. Albeit mitochondria are not required for energy production on fermentative medium, they do contribute to the maintenance of pH homeostasis. The retrograde response, mediated by Rtg1/3, assures mitochondrial integrity. In the event of protein misfolding, the UPR is activated in the ER. Damaged and aggregated proteins are efficiently cleared by autophagy and the ubiquitin-proteasome system (UPS; not shown). (B) Old mother cell. Due to asymmetrical inheritance, mother cells possess an abundance of Pma1 proteins, presumably raising the cytosolic pH, which likely also causes an unavailability of protons to be traversed to the vacuole. The resulting vacuolar alkalization causes insufficient amino acid import by Avt1, leading to mitochondrial dysfunction by a yet unknown mechanism. The retrograde response is activated in an attempt to counteract mitochondrial dysfunction, though it may not be sufficient to maintain mitochondrial integrity. Misfolded proteins accumulate in the ER thereby triggering ER stress, and damaged proteins accumulate in the cytosol due to impaired autophagy and an overwhelmed UPS (not shown). Both reduced mitochondrial integrity and protein misfolding lead to the formation of reactive oxygen species (ROS) and they affect lipid homeostasis thereby further impairing vacuolar functioning. (C) Chronological ageing of young daughter cell. When traversing the diauxic shift, cells experience a disassembly of the V-ATPase and a reduction in Pma1 activity, aided by its endocytosis. As a result, the cytosol acidifies and the vacuole alkalizes. This presumably, similar as in replicatively ageing cells, leads to an impairment of vacuolar amino acid uptake, autophagy, mitophagy and endocytosis. In post-diauxic shift cells, Msn2/4 and Gis1 are activated to induce stress responses and the production of trehalose, which helps to protect cells from acetic acid stress and toxicity of damaged proteins. Although stress responses are optimal in young daughter cells traversing post-diauxic shift, intracellular damage will accumulate over time, eventually surpassing the capacity of the protective machinery, leading to cell demise. (D) Hypothetical model of chronological ageing in old mother cell. In this model we assume a cumulative deregulation of the already decontrolled pH homeostasis in old mother cells following post-diauxic shift. This should lead to an additional impairment of vacuolar amino acid uptake, autophagy, mitophagy and endocytosis and cause a quick accumulation of intracellular damage, leading to more rapid cell demise.