Reviews:
Microbial Cell, Vol. 11, No. 1, pp. 288 - 311; doi: 10.15698/mic2024.08.833
Understanding the molecular mechanisms of human diseases: the benefits of fission yeasts
Department of Genetics and Applied Microbiology, Faculty of Science and Technology, University of Debrecen, Egyetem tér 1, 4032, Debrecen, Hungary.
Keywords: fission yeast, budding yeast, human disease, fungal hyphae, tumor invadopodia, molecular tools.
Received originally: 06/03/2024 Received in revised form: 04/07/2024
Accepted: 10/07/2024
Published: 02/08/2024
Correspondence:
Lajos Acs-Szabo, Department of Genetics and Applied Microbiology, Faculty of Science and Technology, University of Debrecen, Egyetem tér 1, 4032 Debrecen, Hungary; acs-szabo.lajos@science.unideb.hu
Ida Miklos, Department of Genetics and Applied Microbiology, Faculty of Science and Technology, University of Debrecen, Egyetem tér 1, 4032 Debrecen, Hungary; miklos.ida@science.unideb.hu
Conflict of interest statement: The authors declare no competing interests.
Please cite this article as: Lajos Acs-Szabo, Laszlo Attila Papp, Ida Miklos (2024). Understanding the molecular mechanisms of human diseases: the benefits of fission yeasts. Microbial Cell 11: 288-310. doi: 10.15698/mic2024.08.833
Abstract
The role of model organisms such as yeasts in life science research is crucial. Although the baker’s yeast (Saccharomyces cerevisiae) is the most popular model among yeasts, the contribution of the fission yeasts (Schizosaccharomyces) to life science is also indisputable. Since both types of yeasts share several thousands of common orthologous genes with humans, they provide a simple research platform to investigate many fundamental molecular mechanisms and functions, thereby contributing to the understanding of the background of human diseases. In this review, we would like to highlight the many advantages of fission yeasts over budding yeasts. The usefulness of fission yeasts in virus research is shown as an example, presenting the most important research results related to the Human Immunodeficiency Virus Type 1 (HIV-1) Vpr protein. Besides, the potential role of fission yeasts in the study of prion biology is also discussed. Furthermore, we are keen to promote the uprising model yeast Schizosaccharomyces japonicus, which is a dimorphic species in the fission yeast genus. We propose the hyphal growth of S. japonicus as an unusual opportunity as a model to study the invadopodia of human cancer cells since the two seemingly different cell types can be compared along fundamental features. Here we also collect the latest laboratory protocols and bioinformatics tools for the fission yeasts to highlight the many possibilities available to the research community. In addition, we present several limiting factors that everyone should be aware of when working with yeast models.
INTRODUCTION
The use of model organisms to understand essential processes is a well-known strategy in life science research. If we take a look at the publication statistics in repositories like PubMed, we can see that there are a substantial number of studies using different model organisms. For example, a quick search in the aforementioned repository resulted in (accessed on 2023.10.16) 142,277 matches for the keywords ‘Saccharomyces cerevisiae’, 429,254 for ‘Escherichia coli’, 1,945,515 for ‘Mus musculus’, 92,105 for ‘Arabidopsis thaliana’, 62,541 for ‘Drosophila melanogaster’, 36,775 for ‘Caenorhabditis elegans’ just a few to mention. This leads us to conclude that the contribution of model organisms to our understanding of basic biological processes is indispensable.
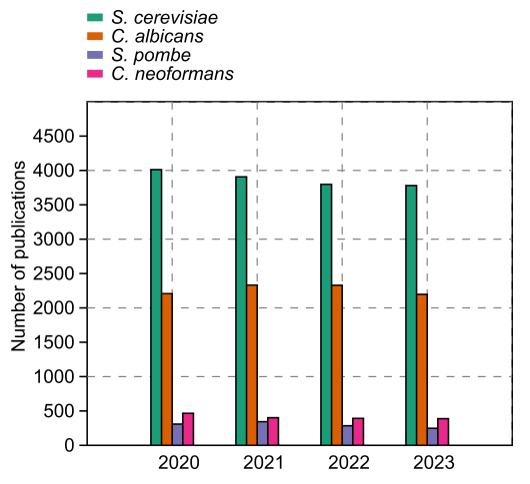
The yeasts have a special place among model organisms because these tiny fungal cells have provided many useful models for different studies. For example, Candida albicans and Cryptococcus neoformans emerged as models for studying fungal pathogenesis, while S. cerevisiae and Schizosaccharomyces pombe are useful models for studying the eukaryotic cell cycle and other countless fundamental biological processes. Accordingly, thousands of research articles related to these species are published every year (Fig. 1). Although S. cerevisiae is the most popular yeast model, we would like to concentrate on the fission yeasts (Schizosaccharomyces) as models in this particular review. The fission yeasts are widely established models of the eukaryotic cell cycle, cell size maintenance, cellular aging, gene expression and epigenetics, autophagy, and apoptotic processes, just a few to mention.
–
To our best knowledge, the fission yeast genus consists of six species to date: S. japonicus, S. pombe, S. octosporus, S. cryophilus, and the recently described species S. osmophilus and S. lindneri, and other variants 1, 2, 3, 4, 5, 6. S. japonicus has two main varieties: var. japonicus and var. versatilis, which have recently been proposed to be considered as two different lineages 7. In our opinion, the two most divergent branches of the genus (S. japonicus and S. pombe) have tremendous potential (not just) as model organisms.
FISSION YEASTS? FOR WHAT?
Someone may ask the legitimate question: why do we need fission yeasts when we already have well-established and widely used yeast models such as S. cerevisiae and C. albicans? What can fission yeasts provide that cannot be provided by the aforementioned ones? Most importantly, could a fission yeast be a good (or better) alternative for studying human diseases than budding yeasts? We try to provide answers to these questions while revealing some fundamental differences among the yeast models (Table 1).
TABLE 1. Fundamental differences of yeast models.
|
Genome stats/ Biological features |
|
|
|
|
|---|---|---|---|---|
|
Genome size (Mb) |
~ 14.28 (SC5314) |
~ 12.24 (S288c) |
~ 12.59 (L972) |
~ 16.6-18.12 (ATCC10660) |
|
Chromosome number (haploid set) |
8 |
16 |
3 |
3 |
|
Chromosome sizes (Mb) |
0.95-3.19 |
0.23-1.55 |
3.5-5.7 |
~ 3.8-5.75 |
|
Coding gene number |
6030 |
5850 |
5134 |
4942 |
|
Common orthologues with humans |
~ 3400 |
~ 3427 |
~ 3422 |
~ 3316 |
|
Disease-associated transcripts |
YTBD* |
~ 1000 |
1521 |
YTBD* |
|
Genetic code |
CTG |
Standard |
Standard |
Standard |
|
Whole Genome Duplication |
pre |
post |
pre |
pre |
|
Preferred chromosomal state |
2n |
2n |
1n |
1n |
|
Centromere sizes |
3-4.5 kb |
125 bp |
35-110 kb |
610-738 kb |
|
Centromere type |
Unique DNA sequence, without repetitive elements |
Small, point-like |
Large, repetitive sequences |
Large, repetitive sequences and transposons |
|
RNAi components |
Yes |
No |
Yes |
Yes |
|
RNAi-mediated splicing |
No |
No |
Yes |
Yes |
|
Percent proportion of introns |
4-6% |
2-6% |
>50% |
>50% |
|
Spliceosome components |
Yes |
Reduced |
Yes |
Yes |
|
Alternative splicing |
Obscure |
Obscure |
Frequent |
YTBD* |
|
Generation time (hours) |
1.7-3.6 |
1.25-2.0 |
2.0-3.0 |
1.0-1.5 |
|
Working time of genetic cross (days) |
Not applicable |
7 |
4 |
2.5 |
|
Pathogenicity |
Yes |
Can be |
No known cases |
No known cases |
|
Hyphae production |
Yes |
Pseudo |
No** |
Yes |
|
Cell division |
Budding |
Budding |
Fission |
Fission |
|
Mitosis |
Closed |
Closed |
Closed |
Semi-open |
|
DNA methylation |
Yes |
No |
No |
YTBD* |
|
H3K9 methylation |
No |
No |
Yes |
Yes |
‘Genome size’ ref.: 8, 9. ‘Chromosome number’ ref.: 10, 11, 12, 13. ‘Chromosome sizes’ ref.: 9, 11, 12, 13. ‘Coding gene number’ ref.:8, 14, 15. ‘Common orthologues with humans’ ref.: 14, 15, 16. ‘Disease associated transcripts’ ref.: 17, 14. ‘Genetic code’ ref.: 18. ‘Whole Genome Duplication’ ref.: 19. ‘Preferred chromosomal state’ ref.: 10, 20, 21, 22. ‘Centromere sizes’ and ‘Centromere type’ ref.: 23, 9, 24, 25. ‘RNAi components’ and ‘RNAi mediated silencing’ ref.: 10, 26, 27. ‘Percent proportion of introns’ and ‘Spliceosome components’ and ‘Alternative splicing’ ref.: 10, 28, 29, 30, 31. ‘Generation time’ ref.: 20, 32, 33, 34. ‘Working time of genetic cross’ ref.: 32, 35. ‘Pathogenicity’ ref.: 36, 37. ‘Mitosis’ ref.: 38, 39. ‘DNA methylation’ ref.: 40, 41, 42. ‘H3K9 methylation’ ref.: 10, 26, 43. * YTBD – Yet to be determined. ** Under standard circumstances, S. pombe does not form hyphae.
Fundamental considerations
At first, we should take a close look at the phylogenies of the fission yeasts. Since they are a basal lineage of the Ascomycota (subdivision Taphrinomycotina), they have a closer phylogenetic relationship with the Metazoa lineage 44, 45, 10, 46, 47. Besides, the fission yeast genus has remarkably conserved common gene content, which is maintained through a relatively long divergence time 10, 48, 49. Maybe that is one of the reasons for them to preserve many common features with the higher eukaryotes. The fission yeasts are already considered “micro-mammalian” model organisms since they share various fundamental features with the metazoan species, such as chromosomal structure and metabolism, relatively large chromosomes and centromeres, low-complexity replication origins, epigenetic mechanisms for regulation of gene expression and centromere maintenance, G2/M control of cell cycle, cytokinesis, mitosis and meiosis, DNA repair and recombination, the mitochondrial translation code, spliceosome components with functional alternative splicing, post-translational modifications, and RNA interference (RNAi) 10, 11, 50, 51, 52, 53.
Chromosomes, centromeres and heterochromatin
The haplontic chromosomal state facilitates genetic modifications and makes the phenotypic association of the mutation more comprehensible. Although both S. cerevisiae and S. pombe are able to maintain haplontic and diplontic chromosomal states as well, in contrast to S. cerevisiae, the fission yeasts preferred the haplontic state. While it seems to be a tendency that the lab strains of S. cerevisiae drive towards diploidization after a few generations, the fission yeasts naturally maintain their haplontic form even in the wild 53, 20, 54, 21, 55, 56, 57. Despite possessing similar genome sizes, S. cerevisiae has many small chromosomes with short (125 bp) point-like centromeres, while the fission yeasts have few but long chromosomes with large centromeres containing repetitive sequences that are more similar to mammalian centromeres 23, 58. Nevertheless, the larger chromosome sizes allow for more efficient microscopic examination. Moreover, the fission yeast genome contains regions of centromeric heterochromatin, which is maintained by H3K9 methylation of nucleosomes and RNAi, unlike the budding yeasts that do not have the necessary molecular toolkit for either one 10, 58, 59, 26, 60. Although both S. pombe and S. cerevisiae have silent chromatin at telomeres, at the mating-type loci, and rDNA regions, only S. pombe has silent chromatin at centromeres 61, 40. In humans, the methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the folate metabolic pathway, loss of function mutations of which are associated with several human conditions, such as cancer, congenital heart disease, and maybe Down and Turner syndrome, too 63, 64, 65, 66. Lim and co-workers examined the fission yeast equivalent of MTHFR, the Met11, and they revealed that it functions to maintain centromeric integrity to ensure precise chromosome segregation in mitosis and meiosis, as the Δmet11 null mutant showed increased missegregation of chromosomes in mitosis and increased transcription from centromeric heterochromatic regions 67. They also observed heterochromatic derepression at subtelomeric and rDNA regions, accompanied by a disruption of H3K9me2 and HP1 protein (Swi6) at all these loci 67. The human nucleosome remodeling and deacetylase (NuRD) complexes sustain specific gene expression programs required for lineage specification, so they have an important role in development and aging 68, 69. In many cases of cancer, the subunits of the NuRD complex contain mutations 70 and some of the mutations can also have detrimental effects on neurological and cognitive development 71. To understand the fundamental function and operation of this heterogenic complex, examination of the fission yeast counterpart Snf2/Hdac Repressive Complex (SHREC) and its interacting partners can be a good alternative 72, 73, 74, 75. Wei and co-workers studied the TOR signaling pathway, and they showed that this cascade targets a conserved nuclear RNA elimination network to dynamically control gene expression by promoting RNA decay and facultative heterochromatin assembly 76. Since RNA elimination factors are involved in proper meiotic progression during oogenesis and/or spermatogenesis in mammals, their result may shed light on the epigenetic reprogramming during development 76, 77, 78, 79. Thus, the fission yeasts proved to be a very powerful model for the investigation of heterochromatin assembly and epigenetic gene silencing 53. Surprisingly, unlike higher eukaryotes, and many other fungal species, neither S. pombe nor S. cerevisiae have DNA methylation processes 40, 41. However, the heterologous expression of a murine DNA methyltransferase in S. cerevisiae resulted in methylated DNA at specific sites 80.
Telomere maintenance
All eukaryotic organisms have precisely defined regions called telomeres at both ends of their chromosomes. Telomere malfunction can cause several problems, from genome rearrangements to several diseases like premature aging, dyskeratosis congenita, and cancer amongst many other diseases 81, 82. One of the protein complexes, the heterotrimeric CST complex plays a key role in the regulation of telomere extension, which can be examined in both the budding and the fission yeast systems 83. The other complex, which contains up to six different proteins, the shelterin-complex has a crucial role in the maintenance of telomeres, as it is responsible for telomere protection and telomerase regulation 83, 84, 85. Strikingly, S. pombe has a shelterin-like telomere complex, which lacks in S. cerevisiae 83, 84. Although the fission yeast shelterin-like complex has “only” three obvious protein orthologues with the vertebrates, the overall structure seems to be quite similar 83, 84, 86, 87, 88, 89. Thus, fundamental processes can be investigated in the fission yeasts also in the case of shelterin function 90, 91, 92. As an example, Irie and co-workers observed in S. pombe that simultaneous inactivation of the shelterin complex subunits Taz1 (TERF1 in humans) and Rap1 (TERF2IP in humans) enables a substantially higher number of gross chromosomal rearrangements per cell division, not just in the telomeric regions but also in the whole genome 93. This is also remarkable because extensive chromosomal rearrangements have been reported in many cancers with mutations in the human shelterin complex 81, 94.
Introns and splicing
Since the fission yeasts have thousands of introns in their genes compared to the few hundred introns of S. cerevisiae, and have degenerate splice site sequences and exonic splicing enhancers, the former species is again a better choice for investigating maturation of mRNA and misregulated splicing 53, 28. Although spliceosome components are available in fission yeasts, functional alternative splicing (AS) has been debated because of the low amount of unequivocal evidence. Montañés and co-workers provided exact proof for functional AS and they showed that it is more prevalent in S. pombe than it was previously thought 29. They have identified 332 alternative isoforms affecting 262 coding genes, 97 of which occur with frequencies >20%. The overwhelming majority of the events (~80%) were intron retention, besides intron inclusion, the use of alternative splicing sites, and exon skipping. According to Zheng and co-workers, the phenomenon of intron retention is one of the least understood forms of alternative splicing in the human genome, even though it can be associated with serious diseases, such as Alzheimer’s disease and cancer 95.
Protein interactions
Thanks to modern sequencing techniques, we were able to identify thousands of mutations associated with diseases and disorders in humans. However, it is still a serious problem to filter out the noise and find the true causes of the observed phenotypes. Moreover, the International Rare Disease Research Consortium (IRDiRC) also acknowledged that different model organisms are an effective experimental system for investigating the impact of gene variants on protein activity, determining their biological function, and identifying potential therapies 96. Thus, yeasts as a system seem to be good candidates for this task too 97, 98, 99. To establish binary protein-protein interactions (PPI) and to find out which mutation causes loss of function or reduced functionality, the yeast two-hybrid (Y2H) system is a well-established method 100, 101, 102. For example, a SARS-CoV2 – human protein interactome was examined in a recent study with the combined usage of Y2H and mass spectrometry 103. Yeasts can also be used for heterologous expression of other eukaryotic proteins, as well as for studying the impact of the foreign protein on the yeast transcriptome and proteome or the effect of different drugs on the proteins to be tested 104, 105, 106, 107. However, these tasks are easier when the interactome of the host is more similar to the tested one. Vo and co-workers created a proteome-wide binary interaction network for S. pombe, and they compared the result with previous data concerning the S. cerevisiae and human interactomes 108, 109, 110, 111. Interestingly, they found that only ~40% of S. pombe interactions are conserved in S. cerevisiae, but ~65% of S. pombe interactions are conserved in humans despite the overall higher sequence similarity between S. pombe and S. cerevisiae 108. Their results therefore suggest that many of the interactions between humans and S. pombe are conserved, but specifically lost in the S. cerevisiae lineage. Besides, they tested whether known disease-causing mutations that disrupt PPIs in humans also disrupt PPIs in S. pombe. Their results showed that in the three tested cases (NMNAT1-NMNAT1, PCBD1-PCBD1, and SNW1-PPIL1), the introduced mutations in the S. pombe counterparts also disrupted PPIs.
Disease-associated genes
The idea that yeast might be a useful model of human diseases has already emerged right after the completion of the sequencing of both S. cerevisiae and S. pombe 11, 12. Based on data from Heinicke et al., S. cerevisiae has approximately 1000 genes, which have orthologues in gene families associated with human diseases 17. In the case of S. pombe, we have an up-to-date and relevant information source on this topic, since the PomBase database is in connection with the Monarch Initiative 112, 113 and Mondo database 114. According to PomBase (https://www.pombase.org accessed on 2024.01.13), S. pombe has 1514 transcripts (proteins and ncRNAs) that are considered orthologues of human disease-associated transcripts 14.
THE ADVANTAGES OF FISSION YEASTS IN VIRUS RESEARCH
Viruses can cause various and often fatal diseases. Effective prevention and treatment of these diseases require extensive knowledge about the molecular mechanism of the infection and the changes caused by the viral proteins in the host cells. Various model organisms are used as hosts to reveal consequences of viral infections. The yeasts belong to these model organisms 115, because of their attractive features, such as eukaryotic cell structure, small genome, widely available molecular tools, and the ability of several eukaryotic viruses to replicate in their cells 20, 116, 117. That is, yeast cells are suitable for heterologous expression and the study of viral proteins.
Here, we would like to provide a brief insight into the research results of viral proteins produced in the fission yeast S. pombe, with particular attention to the Human Immunodeficiency Virus type 1, (HIV-1) Vpr protein, which has been extensively studied in this yeast species.
HIV1 causes Acquired Immunodeficiency Syndrome (AIDS) by damaging the immune system, which is a life-threatening condition. The HIV-1 genome contains several genes, and each protein encoded by these genes has a special role 118, 119. Cloning of these viral genes into S. pombe-specific vectors allowed the researchers to determine the exact cellular localization of the GFP (Green Fluorescent Protein)-tagged viral proteins in the yeast cells 119. The localization of many proteins was revealed for the first time, while further results demonstrated that the intracellular localization of the viral proteins was the same in the yeast and human cells 119.
The Vpr gene (Virus protein R) which encodes a component of virus particles that promotes virus infectivity, has been studied in detail 118. One of the goals was to find out which cellular processes of the host cells are affected by the Vpr protein and whether the same processes are inhibited in the yeast cells and the human cells. Since the S. pombe genomic sequence 14, and the genetic background of its cell processes were well-known, and in addition, a large number of mutant strains were available in this species, it was possible to express the Vpr gene both in the wild-type and various mutant strains. The overproduction of the Vpr gene product revealed that the Vpr protein caused multiple effects on the host cells. The expression of the viral protein resulted in small colonies, growth delay, abnormal cell morphology, arrest in the G2 phase of the cell cycle, and cell death 120, 121, 122, 123, 124. Besides, the Vpr protein caused depletion of the glutathione, and oxidative stress, stimulating the production of reactive oxygen species (ROS) 124, 125, 126. In addition, the direct interaction of the Vpr protein with the proteosome complex, which is responsible for ubiquitin-mediated protein degradation, has also been demonstrated (Fig. 2) 127.
–
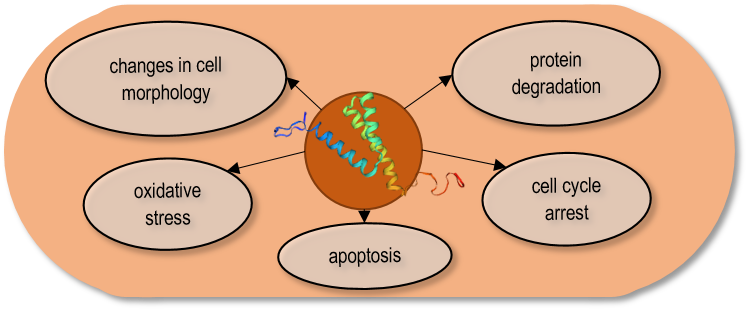 | FIGURE 2: The Vpr protein caused multiple effects on the S. pombe cells. |
To find out how a single viral protein can destroy various cellular processes, the phenotypic changes of the transformed yeast cells were investigated. Examination of cell morphology of the Vpr-transformed cells showed that the changes were caused by several cellular abnormalities, such as disruption of actin cytoskeleton or altered cell polarity 121. Cloning and transformation of the mutant Vpr genes enabled the detection of the effect of a given mutation on the Vpr function. The results obtained in S. pombe showed highly similar changes to the human cells that confirmed the conservation of the Vpr functions. Besides, the truncated genes also revealed that the C-terminal end of the Vpr protein was particularly important for the cell cycle (G2) arrest, while the N-terminal region was required for nuclear localization 128. Chen’s report also demonstrated that the nuclear localization of the Vpr protein was not required for G2 arrest, while it was necessary for cell killing, suggesting that the G2 arrest and cell death caused by Vpr could be independent functions 128.
The investigation of the Vpr-expressing yeast cells shed also light on the molecular background of the cell cycle arrest. The experiments proved that cell cycle arrest correlated well with increased phosphorylation of the Cdc2 kinase, which is the key regulator of mitosis 120, 129. These experiments showed that the regulators of the Cdc2, such as wee1 (encodes an M-phase inhibitor protein kinase) and the cdc25 (encodes a phosphatase, M-phase inducer) were important in the Vpr-induced cell cycle arrest 130, 131. According to the data, the Vpr protein promoted the cytoplasmic compartmentalization of Cdc25 and inhibited its function, which required the Srk1 kinase 123. Since there are differences in cell cycle regulation between S. pombe and S. cerevisiae (the G2/M transition is more important in S. pombe than S. cerevisiae) 53, it was better to choose the fission yeast for the analysis of Vpr-mitosis relation.
The further results also showed that the Vpr protein might affect the cell cycle through different pathways because the rad24 gene (which plays a role in the DNA damage pathway) was also involved in the Vpr-associated cell division defect 131. Based on these results a putative mechanism of the Vpr-induced cell cycle arrest could also be determined 131. The genetic screens, where checkpoint and mitotic regulator mutants were used, have confirmed the complexity of the viral effect, and shed light on the role of further genes, such as rad25, wos2, and hsp16, ef2 that enhanced or suppressed the cell cycle defect or cell death caused by the Vpr protein 124, 130, 132, 133. Examination of Vpr-induced cell death demonstrated that it resembles apoptosis and correlates with changes in mitochondrial morphology. This study described well the pro-apoptotic effects of Vpr 123.
The S. pombe cells were also suitable for finding agents that can reduce the negative effects of the Vpr protein. The H2O2 treatment for example promoted the survival of the Vpr-expressing yeast cells 134, while a simple fission yeast-based screening system allowed to find small molecules that specifically inhibit HIV-1 Vpr 135.
In summary, this simple model organism allowed researchers to reveal the effects of the multifunctional Vpr protein on the host cells. The researchers were able to discover the cellular processes disturbed by the viral protein and their molecular background, while a comparison with the results obtained in mammalian cells showed the conserved characteristics of the viral infection. These results could contribute to a better understanding of the mechanism of viral infection and HIV-1 pathogenesis. Although S. cerevisiae is also used as a model to study the HIV-1 Vpr effects 136, 137, there are some major differences that make S. pombe superior to S. cerevisiae in this regard. Besides the aforementioned cell cycle control point, the Vpr-induced changes in mitochondrial morphology more closely resemble those observed in human cells compared to S. cerevisiae. S. pombe exhibits a greater degree of similarity to humans with respect to mitochondrial features 138. S. pombe displays cell death induced by Vpr that shares some characteristics with apoptosis in human cells, potentially making it a more relevant model for studying this aspect 123.
POMBE FOR PRION BIOLOGY?
Prions are amyloid forms of cellular proteins and are implicated in many incurable and fatal neurodegenerative disorders. Prion disease can be transmitted from organism to organism and is characterized by the accumulation of PrPSc (scrapie isoform of the prion protein). The disease has many forms, such as genetic, sporadic, and acquired 139.
Prions seem to be more widespread than currently appreciated because the research data revealed that yeasts can also have heritable elements transmitted via proteins 140, 141. Since many yeast genome sequences are available, they allow the in silico identification of prion-like genes/proteins in different species 142, 143. In this way, genes with various functions, such as transcriptional regulators, genes involved in sporulation, copper-transport, and translation were identified as prion-associated proteins 141, 143. Structural analyses also showed that asparagine/glutamine-rich domains are linked to amyloidogenesis 140.
In S. pombe 295 PrD (prion-forming domain) containing proteins were identified 143 . One of the prion-like proteins is encoded by the ctr4 gene, the study of which, placed S. pombe on the prion map 143, 144. The overexpressed form of this copper transporter protein was proteinase K-resistant and conferred sensitivity to oxidative stress 143. In addition, overexpression of a S. cerevisiae gene (ScSup35) in S. pombe also demonstrated that this fission yeast can support the formation and propagation of the S. cerevisiae prion 143. Experimental examination of the other genes mentioned above may lead to many new results.
Further characterization of chaperons and heat shock proteins (HSP), as the latter genes are linked to protein folding 139, may reveal especially the new details of prion aggregation. A study has revealed for example that the C-terminal region of HSP104 plays an essential role in prion propagation 145, while the results of Reidy and co-workers confirmed the role of other chaperons in prion propagation 146. As also S. pombe has many hsp genes and genes with GO term “heat shock protein binding” (GO:0031072) (PomBase), their investigation can significantly expand our knowledge of prion disease.
THE DARK HORSE OF EUKARYOTIC CELL RESEARCH: SCHIZOSACCHAROMYCES JAPONICUS
The most divergent branch of the fission yeast genus is the dimorphic S. japonicus 44, 10, 147, which has several features that make it an interesting prospect among other model organisms 32, 148, 149.
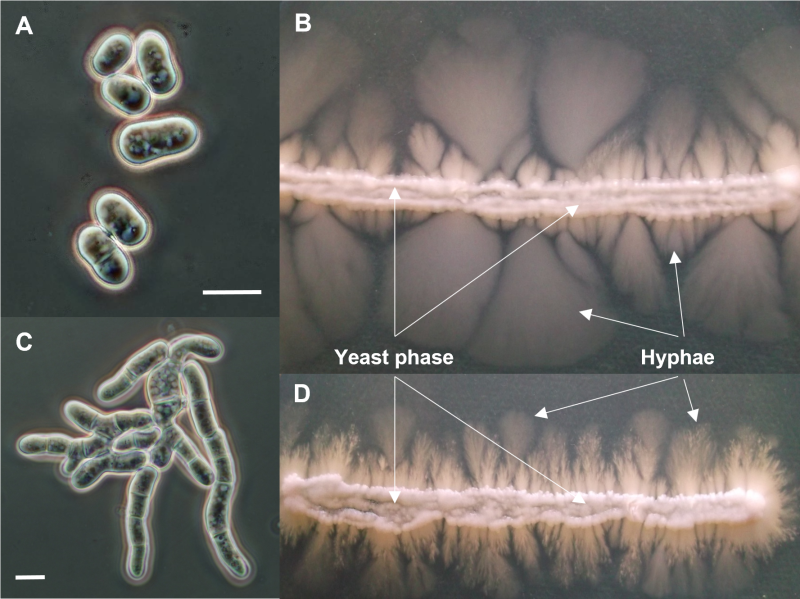
First and foremost, S. japonicus is able to switch between a unicellular yeast form and a true invasive hyphal form 150, 151, 152, 153, 154. Hyphal switching can occur through different stimuli: nutrient deprivation 150, DNA damage 153, 154, the presence of fetal bovine serum (FBS) or fruit extracts 155, 156, and negatively regulated by quorum sensing 157. S. japonicus is not pathogenic to humans, despite its ability to form invasive hyphae that penetrate solid surfaces like agar or gelatine 150, 156, 158. Moreover, hyphal extension is initiated in the presence of FBS even in liquid media, and elevated transcription levels of certain protease-coding genes can be observed in the hyphae 155, 159. Thus, it can be a good non-pathogenic model to study the fungal dimorphism. However, some unique features distinguish it from other dimorphic species such as C. albicans. S. japonicus hyphae does not have a Spitzenkörper, undergoes complete cell divisions, and remains mononuclear 156. Additionally, one of the master regulators of the yeast-to mycelia transition, the transcription factor Nrg1 behaves differently in S. japonicus. In C. albicans, NRG1 represses morphological transition 160, 161, while in S. japonicus, it rather acts as an activator of the hyphal switch 157, 159. Further differences can be observed as nitrogen starvation is a signal that induces a morphological switch in C. albicans, but it is not effective in S. japonicus 162, 163. In this regard, it seems that the MAPK signal transduction pathways contribute somewhat differently to hyphal induction in S. japonicus than in C. albicans 157, 163. Besides, the hyphae of S. japonicus are photoresponsive, which is also an unusual feature among most of the other yeasts 164.
However, S. japonicus is also quite different from its closest relative. While a handful of studies suggest S. pombe can produce adhesive and invasive hyphae-like phenotypes under specific conditions or certain genetic backgrounds 165, 166, 167, 168, 169, 170, S. japonicus remains the definitive dimorphic species within the genus. Furthermore, S. japonicus utilizes a semi-open form of mitosis, while S. pombe undergoes closed mitosis, they differ in the regulation of chromatin-nuclear envelope interactions during mitosis, moreover they exhibit discrepancies in their dynamics of cytokinesis and gene regulation too 171, 172, 38, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186. For example, while S. pombe assembles the actomyosin ring in metaphase and requires a mechanism to prevent its premature constriction, S. japonicus initiates ring assembly only at the mitotic exit, similarly to metazoan cells 149, 176, 187. Although all the fission yeasts have large, centromeric regions with repetitive sequences, S. japonicus does not have specialized pericentromeric repeat sequences as S. pombe has, but it has a larger complement of retrotransposons clustered at centromeric and telomeric regions 10, 60, 188. The S. japonicus centromeres consist of arrays of retrotransposons, which is reminiscent of the human centromeric structure, moreover, the RNAi pathway is indispensable for both S. japonicus and mammalians 188, 189, 190, 191. In S. japonicus, RNAi-mediated silencing of retrotransposons is essential to maintain centromere function and genome integrity, while the other fission yeasts rely on the CENP-B proteins and use RNAi exclusively for heterochromatin maintenance 10, 60, 188, 192. They exhibit discrepancy in their cell-wall composition too: the O-glycans on the cell surface of S. pombe, S. octosporus, and S. cryophilus mainly composed of tetra-saccharides, whereas those of S. japonicus mostly consist of trisaccharides (Gal-Man-Man) 193, 194. Besides, S. japonicus has a wider temperature tolerance: the growth of S. pombe is largely restricted above 37°C, S. japonicus can even grow at 42°C and the generation time is somewhat shorter of S. japonicus than that of S. pombe 32, 148. Strikingly, S. japonicus is well-adapted to anaerobic conditions as it has respiratory deficiency and is able to grow anaerobically without sterol supplementation, which is an unusual ability among eukaryotic organisms 195, 196, 197, 198. In this context, S. japonicus can grow much faster under fermentative conditions than S. pombe, and produces ethanol even at 42°C 197. Alam and co-workers showed that in spite of the fact that S. japonicus does not respire oxygen, it is capable of efficient NADH oxidation, amino acid synthesis, and ATP generation via modification of metabolic pathways 199. S. japonicus is also a suitable model to study membrane bilayer properties and dynamics in anoxic environments, knowing that numerous changes can occur in the membrane lipidomes under hypoxic conditions, for example, in a tumor microenvironment 200, 201, 202.
The phylogenetic distance within the Schizosaccharomyces genus is uniquely large, despite the fact, that they possess remarkably conserved gene content, gene order and gene structure. According to Sipiczki and Rhind et al., at the level of protein sequence identity (~55%), S. japonicus is as distant from S. pombe as the platypus is to humans 44, 10. Interestingly, there might be no genus of Ascomycota that exhibits such a high degree of gene content conservation and sequence divergence at the same time 48, 49, 203. Such sequence divergence, besides the high amount of common gene content, really provides an excellent model pair to study the same cellular processes in different genetic backgrounds. Since most of the laboratory protocols developed for S. pombe can also be used (with slight modifications) for S. japonicus, the parallel investigation of these two species provides an unprecedented opportunity 174, 201, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215.
After we showed that S. japonicus is a remarkable model organism by itself or in comparison with other species, we can ask the question: what is S. japonicus able to bring to human disease research? The answer is not so trivial.
S. JAPONICUS HYPHAL GROWTH AS A MODEL TO STUDY THE INVADOPODIA OF TUMOR CELLS
Besides the above-mentioned advantages of S. japonicus, so far no comparisons have been made between mammalian cells and hyphal growth. This is not surprising because mammalian cells do not form structures such as hyphae, do they? We can say that mammalian cells do not have structures corresponding to hyphae, except for one that resembles its behavior: the invadopodium.
Generally, invadopodia can be described as membrane protrusions, which play a key role in cancer metastasis. These actin-rich structures can reach a diameter of 3 µm and extend several micrometers in length 216. It can digest the surrounding tissues by proteases, to help disseminate the cancerous cells.
One could say that both invadopodia and hyphae are very specialized structures with different roles. However, invasive cell growth may have a deep origin, in which particular features are common among the different lineages 217. Thus, comparisons can be made along five main aspects: polarized growth, actin cytoskeleton, vesicle trafficking, substrate degradation, and environmental sensing (Fig. 3).
–
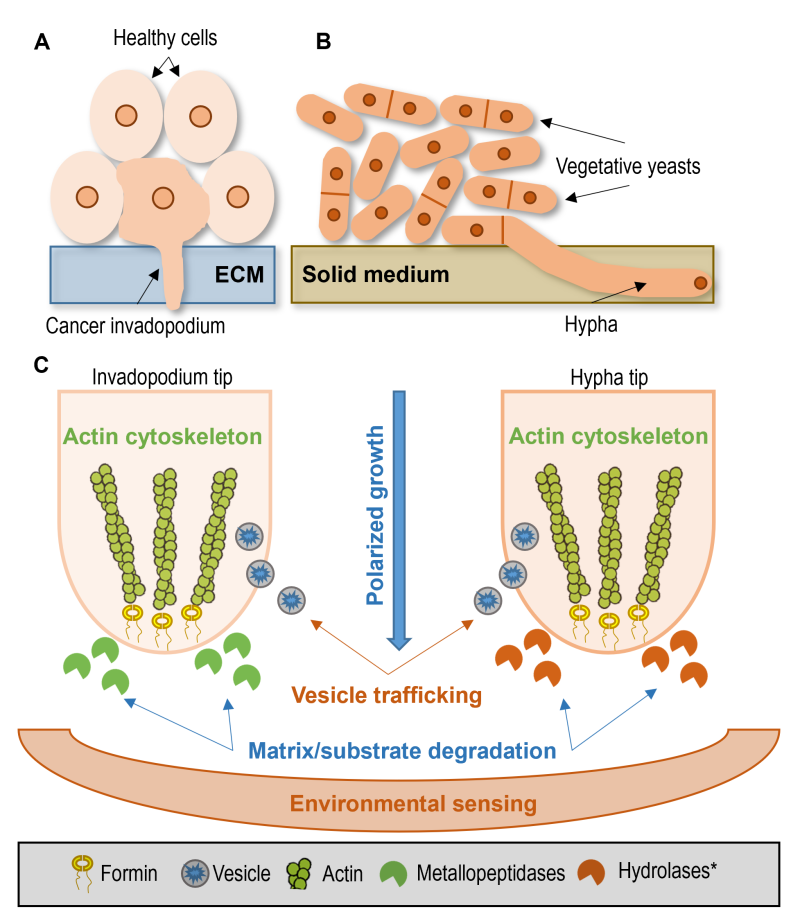 | FIGURE 3: Common features of tumor invadopodia and hyphal growth. (A) Tumor invadopodium invades the extracellular matrix (ECM) among healthy cells. (B) Extension of invasive hypha of the fission yeast S. japonicus in the solid medium among normal vegetative yeast cells. (C) Common features enable a direct comparison between cancer invadopodia (left side) and the fungal hyphae (right side). Polarized growth: both fungal hyphae and invadopodia grow in a polarized way. Actin cytoskeleton: the polarized growth is primarily driven by actin polymerization, and it needs changes in the cytoskeletal structure. Vesicle trafficking: invadopodia formation or hyphae growth is unimaginable without vesicle transport. Matrix/substrate degradation: to continue expansion and acquire nutrition, both the invadopodia and the hyphae need to release enzymes that degrade their surrounding environment. Environmental sensing: signals from the environment have a substantial impact on the behavior of cells. Invadopodia formation and yeast-to-hyphae transition are affected by environmental factors like nutrient availability, pH, temperature, or CO2. *Although transcriptome analysis of the hyphae of S. japonicus suggested that several coding genes responsible for the production of vacuolar hydrolases were upregulated during hyphal extension [159], further studies are required to assess the extent of substrate degradation in S. japonicus. |
Polarized growth
Both fungal hyphae and invadopodia grow in a polarized way. When S. japonicus cells switch from the yeast phase to hyphal growth, they switch from bipolar to unipolar (polarized) growth 151, 156. Similarly, the invadopodium is formed in a specific part of the cell, where the early invadopodium precursors have accumulated 218. The data suggest that this protrusion is often found near the nucleus and Golgi system 219. In addition, not only the position of protrusions themselves but also the polarized exocytosis of matrix metalloproteinases (MMPs) are the indicators of the polarized growth of invadopodia 220, 221, 222. Polarized growth is maintained by the continuous balance of exocytosis and endocytosis 221, and requires an alteration in the actin cytoskeleton in the hyphae too 151, 156.
Actin cytoskeleton
Both invadopodia and hyphae have the same core mechanism, which drives their growth. In the case of invadopodia, the main core structure is F-actin with its regulators (WASP, N-WASP, Arp2/3) 223, 224. The activation of the Arp2/3 complex is a critical step in invadopodia formation, which is responsible for the nucleation of actin 223. Similarly, the accumulation of actin structures at the tips of the growing hyphae was noticed in S. japonicus 151, 156. In addition, Arp2/3 complex activation (presence) was also required for C. albicans hyphae formation 225.
The polarized growth is primarily driven by actin polymerization, which is initiated by the polarisome protein complex 226. Its components play an important role in the polymerization of F-actin into cables, which is required for the proper hyphae formation of C. albicans 226. Similarly, actin polymerization occurs in the invadopodium maturation, in its third step 218. In the case of the fission yeasts, the formin For3 is responsible for actin cable assembly 156, 227. In S. japonicus, actin polymerization is essential for polarized growth as the cells did not show polarized growth at all in the absence of For3 156. Besides, actin depolymerization abolished all vesicle trafficking and cell tip localization of Ypt3 (Rab11 family GTPase), which has a role in cytoskeleton organization 156.
Cortactin, which is another important nucleation-promoting factor, has an important role in the stabilization of the branched actin network and it has a major role in all steps of invadopodia formation 228. Interestingly, the downregulation of cortactin via the p38 pathway resulted in the inhibition of the function and formation of invadopodia in colon cancer 229. In S. japonicus, sty1, which is the orthologue of p38, negatively regulates the induction and progression of hyphal growth 157. The latter indicates that the regulation of certain genes may be similar in invadopodia and hyphae. Therefore, there are critical points that share similarities in mechanism and are also conserved at the gene level.
Vesicle trafficking
Invadopodia formation or hyphae growth is unimaginable without vesicle transport. Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) are key components of vesicle transport which enable fusion between two membranes in an effective and coordinated way, thus allowing delivery of vesicle contents to the target site 230. Gorshtein and co-workers recently reviewed that the inhibition of vesicle trafficking and SNARE family members inhibit invadopodia formation 231.
Similarly, SNARE family members are required for appropriate transport of the cargo vesicles which is essential for hyphal extension and leads to abolished or reduced virulence in pathogenic fungi 230, 232. These data are supported by the strong vacuolization of the S. japonicus hyphae 151, 155, 156. Ypt3 vesicles accumulate at the growing hyphal tips of S. japonicus with a greater intensity than in the yeast cell tips. This probably reflects an increase in membrane trafficking to maintain growth rate of the hyphae 156. In conclusion, the growth of S. japonicus hyphae relies on the transport of vesicles on actin filaments for polarized growth with increased rates of vesicular transport.
Substrate degradation
To continue expansion and acquire nutrition, the hyphae need to release enzymes that degrade their surrounding environment. For example, the C. albicans hyphae produce the secreted aspartyl proteases (SAP), similarly to the cancer cells that digest the host proteins to acquire nutrients 233, 234, 36. In the case of tumor cells, extracellular matrix (ECM) degradation is a crucial step in invading new organs, thus metastasizing 235. Invadopodia release MMPs, which degrade the ECM, facilitating the invasion process 220. It is not clear whether invadopodia formation is also driven by nutrient availability, however, according to van Horssen and co-workers, the metabolic activity of the cancer cell regulates matrix degradation 236.
Although S. japonicus does not have C. albicans-like SAP orthologues, transcriptome analysis of the hyphae of S. japonicus suggested that several coding genes responsible for the production of vacuolar hydrolases were upregulated during hyphal extension (Supplementary Table S1) 159. This finding parallels observations in C. albicans and invadopodia, where hyphal growth is associated with the secretion of hydrolytic enzymes that degrade their surroundings 237, 238, 239. Although further studies are required to assess the extent of substrate degradation in S. japonicus, the elevated expression levels suggest that these enzymes play a role in hyphal elongation, likely in a manner similar to that observed in C. albicans.
Environmental sensing
Signals from the environment have a great impact on the behavior of cells. In most cases, the yeast-to-hyphae transition is affected by nutrient availability, pH, and temperature. In S. japonicus, acidic pH and 37°C, along with different types of nitrogen sources, have a significant impact on filamentous growth 150, 155. In C. albicans, besides the aforementioned factors, CO2 and adherence have triggered filamentous growth 240.
Invadopodia formation is also affected by similar factors, such as pH, CO2, and glucose availability 223, 241. In general, the microenvironment of the tumor plays a crucial role in invadopodia formation and thus in metastasis 242.
TABLE 2. Common orthologues of S. japonicus and humans, whose expression levels are changed in the hyphae and invadopodia in a similar way.
|
|
human gene |
log2 inva |
log2 hyph |
Intersecting GO categories |
|---|---|---|---|---|
|
SJAG_04499 |
P54868 |
2.052 |
0.888 |
acetyl-CoA metabolic process (GO:0006084) |
|
SJAG_03763 |
Q5TDH0 |
1.073 |
0.383 |
proteolysis (GO:0006508) |
|
SJAG_04224 |
Q12788 |
1.049 |
0.288 |
endonucleolytic cleavage to generate mature 5′-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA)(GO:0000472) |
|
SJAG_03961 |
P22557 |
0.964 |
1.126 |
protoporphyrinogen IX biosynthetic process (GO:0006782) |
|
SJAG_03693 |
O95373 |
0.916 |
0.203 |
protein import to nucleus (GO:0006606) |
|
SJAG_01209 |
Q15124 |
0.857 |
0.510 |
carbohydrate metabolic process (GO:0005975) |
|
SJAG_03401 |
Q9BXP2 |
0.813 |
0.941 |
monoatomic ion transport (GO:0006811) |
|
SJAG_03866 |
A0A2R8Y635 |
0.761 |
0.457 |
transmembrane transport (GO:0055085) |
|
SJAG_00451 |
Q9UI42 |
0.757 |
0.476 |
proteolysis (GO:0006508) |
|
SJAG_0230 |
Q9UNX4 |
0.669 |
0.478 |
maturation of SSU-rRNA (GO:0030490) |
|
SJAG_04204 |
P08708 |
0.662 |
0.548 |
translation (GO:0006412) |
|
SJAG_01438 |
P13639 |
0.607 |
0.540 |
translation (GO:0006412) |
|
SJAG_00911 |
Q9BZJ0 |
-0.597 |
-0.718 |
spliceosomal complex assembly (GO:0000245) |
|
SJAG_04821 |
Q96F25 |
-0.603 |
-0.792 |
dolichol-linked oligosaccharide biosynthetic process (GO:0006488) |
|
SJAG_04308 |
Q9NVU7 |
-0.662 |
-0.349 |
ribosomal large subunit export from nucleus (GO:0000055) |
|
SJAG_00272 |
P40938 |
-0.668 |
-0.938 |
DNA replication (GO:0006260) |
|
SJAG_00111 |
Q149N8 |
-0.669 |
-2.141 |
protein polyubiquitination (GO:0000209) |
|
SJAG_01924 |
Q9Y5U8 |
-0.761 |
-0.363 |
mitochondrial pyruvate transmembrane transport (GO:0006850) |
|
SJAG_04540 |
Q13216 |
-0.775 |
-0.604 |
protein polyubiquitination (GO:0000209) |
|
SJAG_04307 |
A0A8Q3WKR8 |
-0.866 |
-0.400 |
isoprenoid biosynthetic process (GO:0008299) |
|
SJAG_03436 |
Q81Y18 |
-0.931 |
-0.605 |
double-strand break repair via homologous recombination (GO:0000724) |
|
SJAG_02625 |
Q14997 |
-0.972 |
-0.452 |
DNA repair (GO0006281) |
|
SJAG_00851 |
O75037 |
-1.080 |
-1.207 |
microtubule-based movement (GO:0007018) |
|
SJAG_16456 |
Q96GW9 |
-1.289 |
-0.795 |
translation (GO:0006412) |
|
SJAG_04068 |
P11168 |
-3.039 |
-0.696 |
carbohydrate transport (GO:0008643) |
|
SJAG_03434 |
P80404 |
-3.543 |
-1.596 |
gamma-aminobutyric acid metabolic process (GO:0009448) |
Each row corresponds to an orthologous protein pair, includes their S. japonicus and human identifiers, and their log2-transformed values, which show their mRNA levels in invadopodia (log2 inva) and hyphae (log2 hyph). Besides, the intersection of Gene Ontology (GO) terms, and the associated GO categories are listed in it. To create this table, we downloaded the human proteome from UniProt (https://www.uniprot.org/ accessed on 12.27.2023.) and the S. japonicus proteome from JaponicusDB (https://www.japonicusdb.org/ accessed on 12.27.2023.). Reciprocal BLAST analysis was carried out using blast+ (ver. 2.13.0) 16. The cutoff value was set to E ≤ 1 × 10-30. Based on these data, we found 1774 common orthologues between S. japonicus and H. sapiens. Then this list was further filtered using the data from 243 and 159, resulting in a total of 86 genes. These genes were subjected to categorization by GO terms (https://www.ebi.ac.uk/QuickGO/help/slims accessed on 12.27.2023.), specifically focusing on the terms associated with biological processes. Only the terms that were present in both species were retained. At last, 26 genes remained, whose belonged to the same GO categories and expressed in a similar way.
Common orthologues and gene regulation
As we have seen in these subchapters, yeast-to-hyphae transition and invadopodia formation have many features in common and are comparable to each other. Despite their distinct functionality, the core mechanisms are very similar. To determine whether these two processes share common genes, we compared the RNA sequencing data from S. japonicus hyphae and invadopodia, without claiming completeness 159, 243. In the case of the S. japonicus hyphae, 1337 genes were significantly upregulated, of which 112 genes showed expression above log2 fold change 2 159. 1484 genes were significantly downregulated, among which 109 had log2 fold changes below 2 159. In the cancer invadopodia, 5873 genes showed elevated expression, while 5467 genes showed decreased expression levels 243. Based on the data of JaponicusDB (accessed on 2024.07.02.), S. japonicus and humans share ~3500 common orthologues (https://www.japonicusdb.org/data/orthologs/). According to our more stringent approach, strict reciprocal BLASTp analyses (E value ≤ 1 × 10-30) revealed 1774 common putative orthologues between S. japonicus and H. sapiens (Supplementary Table S2) 16. The list of common orthologues was compared to the gene lists of the RNA seq obtained from S. japonicus hyphae 159 and human invadopodia 243, which resulted in a total of 85 common genes (Supplementary Table S3). These genes were subjected to categorization by Gene Ontology (GO) terms, considering mainly the common biological processes. In this way, the common orthologue number was reduced to 53 (Supplementary Table S4). 26 genes out of 53 exhibited similar regulation in both hyphae and invadopodia (Table 2), whereas 27 genes showed opposite regulation (Supplementary Table S4). Several gene pairs belonged to the GO categories of transport or metabolic processes (Table 2 and Supplementary table S4). Although the number of common orthologues is quite small and half of the genes were differently regulated, they might still be good indicators or starting points for further investigation of regulatory mechanisms.
We should bear in mind that this is only one pairwise comparison from one-on-one specific conditions, data from different circumstances might result in substantially different gene sets. Moreover, we should also consider the fact that invadopodia are formed in cells that carry severe genetic mutations, in contrast to the normal genetic background of S. japonicus in which the hyphae were produced. Taking these considerations into account, we would consider it particularly interesting to examine the S. japonicus hyphae production in such a mutational genetic background that resembles the genetic background of the invadopodia. In particular because some S. japonicus cell cycle mutant strains produced somewhat different hyphae (Fig. 4 C, D), compared to the wild-type strain (Fig. 4 A, B).
–
LABORATORY USE AND TOOLKITS
Although there is a tendency for most molecular toolkits to be developed for S. cerevisiae first, then adapt to the fission yeasts, sometimes the latter species proves to be a better subject in terms of practical implications. Many wild-type S. cerevisiae strains are used as laboratory models, and because of this, it often happens that the same mutation causes different phenotypes in different wild-type strains 244, 245. This often makes the comparison of the results difficult. In contrast, almost every lab working with S. pombe uses the same strains: the L968 h90 homotallic, the L972 h– and the L975 h+ heterotallic strains isolated by Urs Leupold 246. As a result, majority of the studies using S. pombe can be directly compared and contrasted. Furthermore, L968 is a natural isolate, which does not behave differently compared to the other natural isolates 247.
Numerous useful databases, protocols and toolkits have arisen through the years for the fission yeasts. Virtually, all the molecular biology tools available were adapted or can be adapted to fission yeasts, from standard gene replacements to CRISPR-Cas9 and from FISH to Hi-C systems 248, 249, 250, 251, 252. Since Herrera-Camacho and co-workers have presented many useful applications for S. pombe, here we just focus on the recently described methods, online tools and algorithms (Table 3 and 4) 251.
TABLE 3. Online platforms and bioinformatic tools for the fission yeasts.
|
Databases/Tools |
Links |
|
|---|---|---|
|
Pombase |
||
|
Forsburg lab |
||
|
Bähler lab |
||
|
EnsemblFungi |
http://funig.ensembl.org/Schizosaccaromyces_pombe/Info/Index |
|
|
EnsemblFung |
https://funig.ensembl.org/Schizosaccaromyces_japonicus/Info/Index |
|
|
JaponicusDB |
||
|
Oliferenko lab |
||
|
Japonet |
||
|
Methods/Tools |
Description |
Reference |
|
Protein function prediction |
Phenomics and machine-learning approaches to predict protein function |
|
|
pomBseen |
Analysis pipeline for the quantitation of fission yeat micrographs containnig bright-field channel and up to two fluorescent channels |
|
|
DeepEdit |
A powerful tool for the study of RNA editing |
|
|
DeePiCt |
An open-source deep-learning framework for supervised segmentation and macromolecuar complex localization in cryo-electron tomography |
|
|
3D models of chromosomes |
Building 3D models from raw Hi-C data |
|
|
YEASTRACT+ |
Tool for the analysis, prediction and modelling of transcription regulatory data |
|
|
PTMint |
Manually curated complete experimental evidence of the PTM regulation on protein-protein interactions |
|
|
Metabolic modelling |
Computational modeling of metabolic networks |
|
|
Photo Phenosizer |
Machine learning-based method to measure cell dimensions |
|
|
3D-SIM pipeline |
Three-dimensional structured illumination microscopy (3D-SIM) image analysis pipeline for nuclear pore complex quantitation |
|
|
Serine phosphorylation prediction |
A computational predictor was proposed to predict serine phophorylation sites mapping on S. pombe |
|
|
Yesprit and Yeaseq |
Applications for designing primers and browsing sequences in four fission yeast species |
|
|
GproDIA |
A framwork for the proteome-wide characterization of intact glacopeptides from data independent acquisition (DIA) data with comprehensive statistical control |
|
|
Spindle elongation dynamics |
An ImageJ plugin that can automatically track S. pombe spindle length over time and replace manual or semi-automated tracking of spindle elongation dynamics |
|
|
ChroMo |
An interactive, unsupervised cloud application specifically designed for exploring chromosome movement datasets from live imaging |
|
TABLE 4. Recent experimental tools and protocols for the fission yeasts.
|
Methods/Tools |
Description |
References |
|---|---|---|
|
Quantifying turgor pressure |
Experimental approach to access turgor pressure in yeasts based upon the determination of isotonic concentration using protoplasts as osmometers |
|
|
POMBOX |
Modular tools for generating plasmids with up to 12 transcriptional units |
|
|
New vectors |
New S. pombe vector systems employing lys1 and arg3 as markers |
|
|
CRISPRi |
CRISPR interference method to study essential genes in S. pombe |
|
|
BiFCo |
Introduction of bimolecular fluorescent cohesin to monitor cohesin complex assembly and disassembly |
|
|
CRISPR-Cas13d |
Implementation of the CRISPR-Cas13d system in fission yeast for RNA knockdown |
|
|
Kinetochore nanostructure |
Construction of a nanometer-precise in situ map of the human-like regional kintetochore of S. pombe using multi-color single-molecule localization microscopy |
|
|
Heterothallic strains |
Creating heterothallic strains of S. pombe |
|
|
SLIPT |
Introduction of self-localizing ligand-induced protein translocation (SLIPT) system in S. pombe |
|
|
SILAC |
Stable isotope labeling by amino acids (SILAC) to apply for protein identification and quantification |
|
|
TCP-seq |
Translation-complex profiling of fission yeast cells |
|
|
Visualizing tropomyosins |
Tools to visualize tropomyosins in four different organisms/cell types using an mNG fusion strategy |
|
|
Barcoded mutant arrays |
Construction of a S. pombe transposon insertion library |
|
|
Mulitcopy suppressors |
A protocol for carrying out ‘multicopy suppression’-based genetic screen in S. pombe |
|
|
Fluorescnece exclusion |
A rapid, accurate and powerful method for measuring yeast cell volume |
|
|
Protein-RNA interactions |
Quantitative analysis of protein-RNA interactions |
|
|
DNA Curtain Technique |
DNA curtain is a hybrid technique that combines lipid fluidity, microfluidics, and total internal reflection fluorescence microscopy (TIRFM) to provide a universal platform for real-time imaging of diverse protein-DNA interactions |
|
|
PDE inhibitors |
Platform for expressing cloned cyclic nucleotide phosphodiesterases (PDEs) and robust screening for small molecule inhibitors that are cell permeable |
|
|
Cell cycle stage |
Detecting cell cycle stage and progression in fission yeast |
|
|
Cell cycle synchrony |
Cell cycle synchrony methods for fission yeast |
|
|
Mitotic inheritance of histone modifications |
A framework to successfully implement an inducible heterochromatin establishment system and evaluate its molecular properties |
|
|
DRIP assay |
Antibody-based DNA:RNA immunoprecipitation (DRIP) strategy |
|
|
Near-infrared imaging |
Easy use of multiplexed live-cell imaging in fission yeast with a broader color palette |
|
|
Protein interactions |
Introduction of an efficient and convenient method termed the Pil1 co-tethering assay to detect binary, ternary, and quaternary protein interactions |
|
|
Local protein accumulation kinetics |
A detailed protocol for determining protein accumulation kinetics at the division site in S. pombe and S. cerevisiae |
|
|
G-Quadruplex-DNA-Disrupting Small Molecules |
In vitro assays to reliably identify molecules able to destabilize G-quadruplex-DNA |
|
|
New vectors |
New vectors to simplify the genome editing protocols |
|
|
Hyphal RNA isolation |
Simple method to grow hyphae and isolate quality RNA from hyphal tips |
|
|
AID vectors |
Two plasmids that facilitate the introduction of the mini auxin-inducible degron (mAID) tag with a FLAG epitope or GFP by the conventional PCR-based gene targeting method |
Both S. pombe and S. japonicus have their own dedicated databases: PomBase and JaponicusDB, which are community-curated (Table 3) 14, 15. These platforms summarize the results reported by the researchers working with the fission yeasts; they enable a rapid overview of the recent developments in many topics.
LIMITATIONS
Despite all good features of yeasts, they also have their own limiting factors. Since all the fission yeast species have their unique elements of metabolic pathways and protein interaction networks, most of the biological processes can only be “similar” to their human counterparts. Although we could gain useful information about human diseases using fission yeast models 297, 298, 299, 300, 301, 302, 303, 304, 305, there will always be differences that we should be cautious about. At the same time, complex processes cannot be investigated because of the lack of multicellular phenotypes. But beyond the trivial, there are other factors to consider.
The creation of auxotrophic mutant strains is a widely used procedure in yeast genetics. Auxotrophic mutant strains enable researchers to easily verify the success of a gene deletion or plasmid vector introduction into the cells, for example. However, there is emerging evidence that a knockdown of even a simple metabolic gene could produce a pleiotropic effect, which causes a complex phenotype leading to false conclusions. For instance, a defect in certain amino acid (AA) biosynthetic pathways may activate the general AA control and suppress the TOR pathway, depending on the growth conditions 306, 307, 308. In the case of S. pombe, leucine (Leu) auxotroph strains have been used for decades 309, although Leu auxotrophy can cause altered intracellular response compared to the prototrophic wild-type strain 307. Similarly, the use of the URA3 gene as a selective marker caused decreased virulence in C. albicans, thus, it resulted in misleading phenotypes 310, 311. The effect could be more severe in the case of strains that have two or more auxotrophies. The situation is not much better when using antibiotic-resistant genes as genetic markers. To ensure a sufficient expression of the marker gene, constitutive promoters are generally used. Those promoters sometimes act bidirectional or might elevate the expression levels of the neighboring genes too 312. In that particular case, we are again facing a pleiotropic phenotype. It is also common practice to knock out one of the members of the non-homologous DNA end joining (NHEJ) repair system to enhance gene targeting efficiency. Without efficient NHEJ, the cells ideally use the homologous recombination repair system, which enables precise integration of the foreign DNA into the target genome. Despite NHEJ-deficient strains showing normal phenotypes in standard circumstances, NHEJ members have many other roles that go beyond just joining DNA ends. In S. pombe, the Pku70-Pku80 heterodimer plays a critical role in telomere length maintenance and recovery from replication stress 313, 314. In S. japonicus, disruption of the Ligase 4 (lig4) gene resulted in a seemingly normal phenotype 315. However, increased sporulation on complete medium, decreased hyphal growth, faster chronological aging, and higher sensitivity to heat shock, UV light, and caffeine can be observed in the lig4-disrupted strains 315. Thus, gene characterization conducted in an NHEJ-deficient strain could also lead to incorrect conclusions.
Although we are able to track dynamic cellular processes by using chemical inhibitors, many drugs are not efficient enough for fission yeasts because of their multidrug resistance (MDR) 316, 317, 318. Thus, finding a new therapeutic agent or investigating the performance of a candidate might not work well in the fission yeast (or in other yeasts). However, there are several counterexamples, of course 319, 320. It is even possible to make fission yeasts sensitive to drugs by engineering their MDR-related genes 318, but the effect of those gene deletions might resemble the gene deletion effects of other metabolic or DNA repair pathway genes.
In fact, any gene knockdown could cause secondary gene mutations or overall genomic imbalance, which initiates adaptive genomic changes 321. Of course, the purpose of many studies is exactly to understand these changes. But we should bear in mind, that all the yeasts have a very short generation time, and the continuous inoculation of their cells could result in multiple bottlenecks and in parallel, forced genome evolution. There is a lot of anecdotal evidence circulating among researchers when they experience that a mutant yeast strain completely changes its behavior after a certain number of rounds of inoculation. To provide experimental evidence, Szamecz et al. showed that many knockout strains have recovered and exhibited almost as good fitness as the wild-type strain did after certain rounds of generation 322. Although they performed their experiments with S. cerevisiae, their result could easily be true for the fission yeasts too.
CONCLUSIONS
With this particular review, we would have liked to emphasize the importance of the fission yeast models, as we are convinced that they still have many unexploited benefits. Although the number of researchers using fission yeasts is relatively high, the size of the fission yeast community is nowhere near the size of the budding yeast community. Obviously, in many cases, it is easier to work with S. cerevisiae, but the fission yeasts share substantially more fundamental biological processes with the metazoans. Therefore, we wanted to highlight some of the differences between budding and fission yeast models, without claiming completeness. Besides, we also wanted to promote S. japonicus as a less-known, but emerging model organism with unique features. As we have shown, S. japonicus is more similar to mammalian cells in certain features than S. pombe is. Moreover, in our view, S. japonicus can easily be a non-mammalian model for tumor invadopodia studies, since the fundamental processes of invadopodia formation and hyphae formation can be rationally compared. Although it is obvious that none of the yeasts can be used as equivalent models of human diseases, we believe that the fission yeasts could substantially contribute to our understanding of the molecular background of human diseases.
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
The authors of this review apologize to the many authors whose articles have not been cited for reasons of length. Laszlo Attila Papp received funding from the project TKP2021-EGA-18. Project no. TKP2021-EGA-18 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. This publication was supported by the University of Debrecen Program for Scientific Publication.
COPYRIGHT
© 2024

Understanding the molecular mechanisms of human diseases: the benefits of fission yeasts by Acs-Szabo et al. is licensed under a Creative Commons Attribution 4.0 International License.