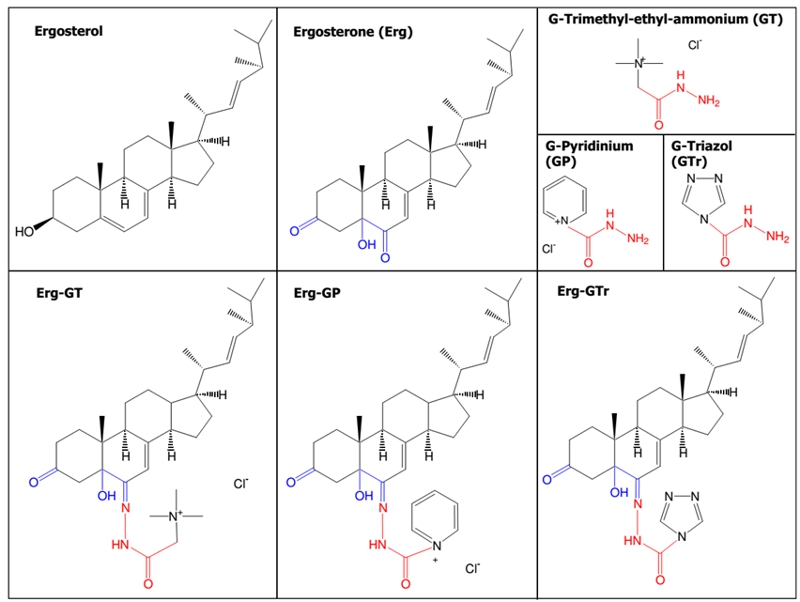
FIGURE 1: Structures of the compounds evaluated on Leishmania mexicana promastigotes.
IUPAC names: Ergosterol: 10,13-dimethyl-17-(1,4,5-trimethyl-hex-2-enyl)-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; Ergosterone (Erg): 5-hydroxy-10,13-dimethyl-17-(1,4,5-trimethyl-hex-2-enyl)-1,4,5,9,10,11,12,13,14,15,16,17-dodecahydro-2H-cyclopenta[a]phenanthrene-3,6-dione; GT: hydrazinocarbonylmethyl-trimethyl-ammonium chloride; GP: 1-hydrazinocarbonyl-pyridinium chloride; GTr: [1,2,4]triazole-4-carboxylic acid hydrazide; Erg-GT: [5-hydroxy-10-methyl-3-oxo-17-(1,4,5-trimethyl-hex-2-enyl)-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-6-ylidene-hydrazinocarbonylmethyl]-trimethyl-ammonium chloride; Erg-GP: 1-[5-hydroxy-10-methyl-3-oxo-17-(1,4,5-trimethyl-hex-2-enyl)-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-6-ylidene-hydrazinocarbonyl]-pyridinium chloride; Erg-GTr: [1,2,4]triazole-4-carboxylic acid [5-hydroxy-10,13-dimethyl-3-oxo-17-(1,4,5-trimethyl-hex-2-enyl)-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-6-ylidene]-hydrazide. The global yield of the synthesis was 49%, 45%, and 49% for Erg-GTr, Erg-GT, and Erg-GP, respectively.