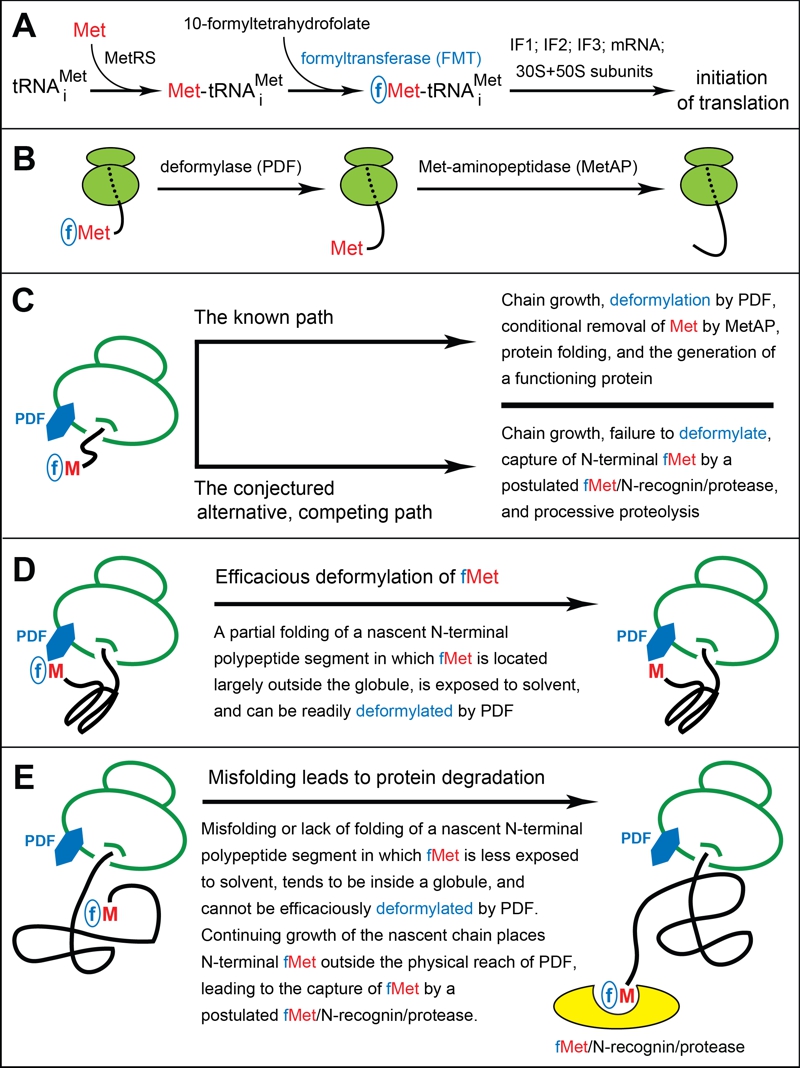
FIGURE 1: The working model of fMet/N-degrons.
(A) Pretranslational enzymatic steps that result in formyl-Met (fMet) becoming the first residue of a nascent bacterial polypeptide. MetRS, Met-tRNA synthetase. IF proteins, initiation factors.
(B) Translating ribosomes, with reversibly associated (not depicted) deformylase (PDF) and Met-aminopeptidase (MetAP) competing for their overlapping binding sites near the exit from the ribosomal tunnel. High‑affinity binding by the TF chaperone to a nascent polypeptide chain occurs once its length exceeds ~100 residues, usually after deformylation of N-terminal Met. A nascent chain, depicted unfolded in this diagram, tends to become unstably folded as it emerges from the tunnel. The rate of the MetAP-mediated removal of the deformylated N‑terminal Met residue depends on the identity of a residue at position 2.
(C-E) Self-explanatory descriptions of the working model of fMet/N-degrons. See the main text for additional details and relevant citations.