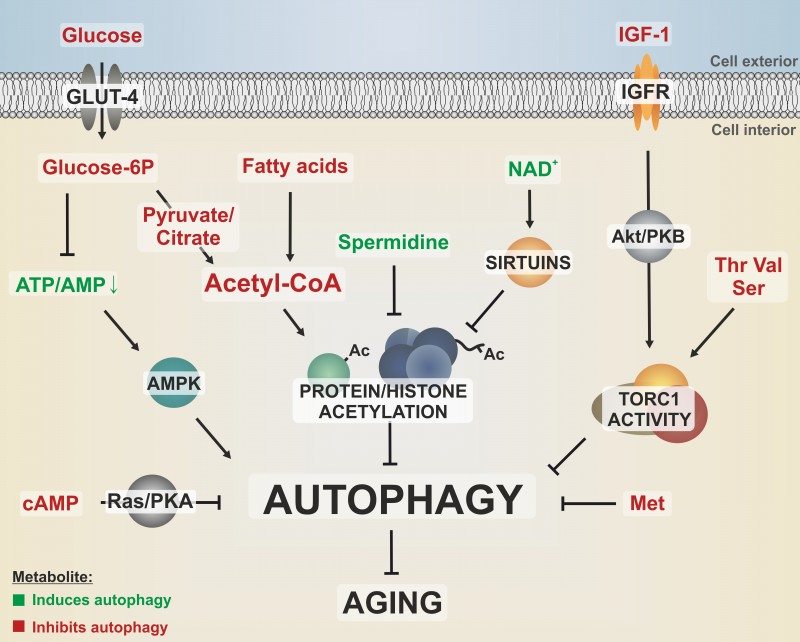
FIGURE 1: Different metabolites converge on pathways that regulate autophagy and aging.
Dietary nutrients like glucose, amino acids and fatty acids as well as growth signaling by IGF-1 activate nutrient-sensing kinases, like the target of rapamycin complex 1 (TORC1), which stalls autophagy via phosphorylation of downstream targets. Furthermore, autophagy is negatively regulated by the Ras/PKA pathway, which responds to nutrient availability by sensing intracellular cAMP levels. The cellular energy status is reflected by the ATP/AMP ratio, which is sensed by the autophagy activator AMPK. Methionine downregulates autophagy during aging in a yet to be elucidated fashion. The central energy intermediate acetyl-CoA integrates metabolites from glycolysis, β-oxidation or respiration and fuels acetylation of proteins such as histones, resulting in decreased autophagic flux. All these autophagy-limiting metabolic pathways have been linked to an accelerated aging phenotype. In contrast, polyamines, like spermidine, reduce protein acetylation, thereby promoting autophagy and longevity. Potential crosstalks between protein acetylation and nutrient sensing kinase signaling are yet to be elucidated. GLUT-4, glucose transporter 4; IGFR, IGF-1 receptor; Ac, acetyl-group; amino acids are indicated by three-letter code.